- Biomedical Research (2010) Volume 21, Issue 3
Prevalence of genital Chlamydia trachomatis in women using PCR on urine specimen
Vineeta Mittal1*, Jyotsna Agarwal, Amita Jain, Anoop K Verma1Department of Microbiology, CSM Medical University, Lucknow, India.
- Corresponding Author:
- Vineeta Mittal
Departments of Microbiology
Era’s Lucknow Medical College
Lucknow 226003, India
Accepted date: December 15 2009
Abstract
Aim of present study was to determine prevalence of genital Chlamydia trachomatis in women of reproductive age group using PCR on urine specimen. First void 30 ml urine specimen from 160 women attending gynaecology OPD at KGMU, Lucknow were subjected to DNA extraction and PCR. 90 women had symptoms suggestive of genital tract infection (cases) and remaining 70 women were symptom free and served as control. An in- house PCR test by KL-1 and KL-2 plasmid primers was standardized on known positive urine specimen. Statistical analysis was done by chi square test. In our study, prevalence of genital Chlamydia trachomatis was 9.3%, it was more in cases (11.11%) in comparison to controls (7.14%) ( P >0.10). The difference between symptomatic and asymptomatic women is not statistically significant so we recommend universal screening of Chlamydia trachomatis in general population.
Keywords
Chlamydia trachomatis, polymerase chain reaction, first void urine
Introduction
Chlamydia trachomatis is exclusively a human pathogen and is one of the most common sexually transmitted pathogen in United States with an estimated 4.5 Million new cases each year. It is common cause of urethritis and cervicitis. Infection with this agent can be asymptomatic in up to 80% of women which can make diagnosis and detection difficult [1] Left undetected and untreated chlamydia can ascend the upper genital tract causing inflammation and scarring in both male and female reproductive tract and causes serious sequelae including PID, infertility, ectopic pregnancy, perihepatitis in females and epididymitis, proctitis and arthritis in men [2] The agent in the cervix may be transmitted to the neonate passing through the infected birth canal and an eye infection (inclusion conjunctivitis of the newborn) and chlamydial pneumonia of infants may develop [3]. Chlamydial genital tract infections have been associated with increased rates of transmission of HIV [4]. Therefore screening for genital chlamydia infection is a high public health priority.
Reliable data on the prevalence of genital Chlamydia infection in India is not available. Using conventional serologic and antigen detection assays, a prevalence ranging from 30% to as high as 81% has been reported for chlamydia in asymptomatic women.
Although many diagnostic modalities are present for detection of Chlamydia trachomatis, none are 100% sensitive. Each has its own limitations. Of the available techniques, Nucleic acid amplification tests are most sensitive and specific. Polymerase Chain Reaction is an accurate, rapid and reliable method for the detection of Chlamydia trachomatis [5]. Polymerase Chain Reaction can be done on noninvasively collected specimens i.e. first voided urine sample (FVU). This allows their use in screening asymptomatic individuals who represent the bulk of prevalent infections [1] .Thus the aim of this study was to determine the prevalence of genital Chlamydia trachomatis infection in sexually active asymptomatic and symptomatic women using an 'in-house' Polymerase Chain Reaction on urine specimen
Subjects and Methods
This study was carried out in the postgraduate Department of Microbiology, KGMU, Lucknow. Clinical samples were taken from OPD of obstetrics and gynaecology, KGMU, Lucknow between October 2003 to September 2004.
Total 160 clinical samples were taken. Of which 90 samples were from symptomatic females with vaginal discharge and 70 samples were from asymptomatic females.
Inclusion Criteria
Sexually active women (15-45 years) attending general Gynae, out patient department, having pathological vaginal discharge acting as cases.and sexually active women (15-45 years) with no complaints suggestive genital infection such as vaginal discharge, perineal itching or lower abdominal pain acting as controls. Informed written consent to participate would be obtained from each patient.
Exclusion Criteria : Sexually in active women i.e.<15 years or > 45 years, pregnancy, women who were menstruating, women with cancerous or precancerous lesion of cervix and history of antibiotics intake in last 10 days. Stastistical analysis was done by Chi square test
Laboratory Techniques
Specimen
First void urine (FVU) ~ 30 ml (first part of the stream catch during any time of the day) would be collected in sterile plastic containers and would be transported to the laboratory as early as possible. In the lab centrifuged ~30ml of urine at 3000 rpm for 20 minutes, discarded the supernatant and deposit was aliquoted in triplet and stored at –20°C till further processing. The detection of Chlamydia trachomatis would be done by PCR.
DNA Extraction
Step 1: 500 μl of FVU sediment centrifuge at 12000 rpm for 20 minutes in a microcentrifuge at room temperature. Discard the supernatant
Step 2: Add 500 μl of TDW, vortex, centrifuge at 12000 rpm for 10 minutes, discard the supernatant .Repeat step 2 once again
Step 3: Add ~ 300 ml of lysis buffer to deposit ,vortex properly, incubate in boiling water bath for 20 minutes., cool and add 10μl proteinase K (10μg/ μl),vortex ,incubated at 55°C in water bath for 2 hour and in last incubate at 100°C in water bath for 15 min to inactivate proteinase K.
Step 4: Purification of DNA by Phenol Chloroform extraction and DNA precipitation by ethanol.
Step 5: Purity Check of Extracted DNA was checked by spectrophotometer
Amplification
A pair of chlamydia trachomatis specific oligonucleotide primers KL1 and KL2 [6] based on cryptic plasmid (Synthesized by Bangalore Genei India) was used. Amplified product size is 241 bp. Primers have following base sequences KL1, 5' – TCCGGAGCGAGTTACGAAGA-3 KL2, 5' – AATCAATGCCCGGGATTGGT-3'
Amplification reaction were performed in a final volume of 20 μl. 5 μl of extracted DNA was added to 15 μl of PCR mixture containing
d NTP (100μM) = 0.8 μl (100 μM each dATP, dGTP, dCTP, dTTP) Primers KL1 (0.01 μM) = 0.24 μl (24 pmole) Primers KL2 (0.01 μM) = 0.24 μl (24 pmole) Taq Polymerase (3 U) = 0.3 μl (0.9 U) PCR buffer 10x = 2.0 μl (1x) Dissolve in 11.42 μl of TDW.
Note: dNTP, Taq polymerase & PCR buffer (Synthesized by Bangalore Genei India)
Amplification mixtures were subjected to 35 cycles of amplification on automated thermocycler (TECHNE, porgene)
Amplification cycle as followed Initial denaturation for 5 min at 94°C Next 35 cycles for 1 min at 94°C , 2 min at 50°C and 3 min at 72°C
Last extension for 10 min at 72°C
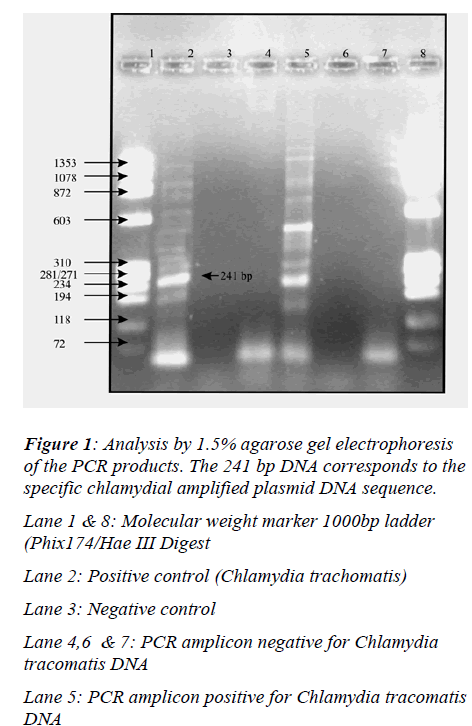
Analysis of amplified product by 1.5% agarose gel electrophoresis (Figure 1)
Results
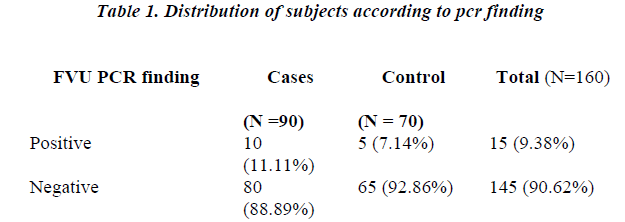
The study group comprised of 160 subjects of sexually active age group (15-45 years) attending the gynaecology out patient department. Ninety women presented with symptoms suggestive of genital tract infection which included vaginal discharge, with or without perineal itching, lower abdominal pain or urinary complaints. These were taken as cases. Seventy women attending the family planning clinic, infertility clinic or coming for contraceptive counseling or follow through cases of abortion or delivery and laparoscopy ligation were taken as controls. They were without any symptom suggestive on genital infection. There were 10 (11.11%) positive cases as against 5 (7.14%) positive controls according to FVU PCR findings. (Table 1)
Lane 1 & 8: Molecular weight marker 1000bp ladder (Phix174/Hae III Digest
Lane 2: Positive control (Chlamydia trachomatis)
Lane 3: Negative control
Lane 4,6 & 7: PCR amplicon negative for Chlamydia tracomatis DNA
Lane 5: PCR amplicon positive for Chlamydia tracomatis DNA
Discussion
In our study, the prevalence of genital chlamydia infection was found to be 9.3%. The prevalence was more among symptomatic population (11.11%) in comparison to asymptomatic population (7.14%). ( P>0.10). In the past studies in India, the prevalence among women having vaginal discharge was reported to be 12.2% by using conventional serological methods [7]. Malathi et al., (2002) also reported the prevalence of Chlamydia trachomatis in women with mucopurulent cervicitis as 4% by using PCR on endocervical swabs [8] in India. Anova et al., found Chlamydia trachomatis infection in 8% of women attending for termination of pregnancy [9]. Quinn. et al., demonstrated the prevalence of Chlamydia trachomatis was 12.8% in women attending STD clinic [10]. Toye B et al., showed the prevalence of Chlamydia trachomatis infection in women to be 7.9% [11]. Bontis, Vavilis, Panidis [11] has found the overall prevalence of CT infection of 4% in asymptomatic women [12]. Paukku, Puolakkainen, Apter [12] has seen the prevalence of 5.6% in asymptomatic women by FVU PCR assay [13].
Conclusion
In our study this difference between symptomatic and asymptomatic women is not statistically significant so we recommend universal screening of Chlamydia trachomatis in general population.
References
- Watson EJ, Templeton A, Russel I, et al The accuracy and efficacy of screening tests for Chlamydia trachomatis: a systemic review. J Med Microbiol; (2002.) 51: 1021-1031.
- Singh V, Rastogi S, Gang S, et al PCR for detection of endocervical Chlamydia trachomatis infection in women attending a Gynecology out patient department in India(2002). Acta Cytologica; 46: 540-544.Harrison HR, Alexender ER, chlamydial infections in infants and children. In: Holms KK, Mardh PA, Sparling PF et al., eds. Sexually transmitted diseases 2nd ed. New York, McGrow Hill1990,811-820
- Wasserheit JN, effect of changes in human ecology and behavior on patterns of sexually transmitted diseases including HIV infection, Proc. Natl. Acad .Sci. USA 1994,91,2430-2435
- Simko J, Holly I, Hudecova M, Zaviacic T, Holoman K, Diagnosis of Chlamydia trachomatis using PCR in Gynaecology patients in Slovakia. Ceska Gynekol 002; 67(6) 376-379.
- Mahony JB, Kathleen E Luinstra, John W sellers, et al. Confirmatory polymerase chain reaction testing for Chlamydia trachomatis in first-void urine from asymptomatic and symptomatic men. J. Clin. Microbiol. 1992: 2241-2245.
- Vishwanath S, Talwar V, Prasad R, Coyaji K, Elias CJ, de Zoysa I. Syndromic management of vaginal discharge among women in a reproductive health clinic in India. Sex Transm. Infect. 2000; 76: 303-6.
- Malathi J, Madhavan HN, Therese KL, Rinku JP, Narendar KP. Prevalence of Chlamydia trachomatis and herpes simplex virus in males with urethritis and females with cervicitis attending STD clinic. Indian. J. Med. Res. 2002; 116: 58-63.
- Anova L Blackwell, Philip D Thomas, Kathie Wareham et al ,Health gains from screening for infections of the lower genital tract in attending for termination of pre gnancy.Lancet1993;342: 206-10.
- Quinn TC, Welsh L, Lentz Andrew, Crotchfelt KY, Zenilman J, Newhall James, Gaydos C, (1996). Diagnosis by AMPLICOR PCR of Chlamydia trachomatis infection in urine samples from women and men attending sexually transmitted disease clinics. J Clin Microbiol, 34; 1401-1406
- Toye B, Peeling RW, Jesamine P, Claman P, Germmill I. Diagnosis of Chlamydia trachomatis infections in asymptomatic men and women by PCR assay. J. Clin. Microbiol 1996; 34(6): 1396-1400.
- Bontis J, Vavilis D, Panidis D. Detection of Chlamydia trachomatis in asymptomatic women: relationship to history, contraception and cervicitis. Adv. Contracept. 1994 Dec; 10 (4): 309-15.
- Paukku M, Puolakkainen M, Apter D. First void urine testing for Chlamydia trachomatis by polymerase chain reaction in symptomatic women. Sex Transm. Dis. 1997 July; 24(6); 343-346.
- Watson EJ, Templeton A, Russel I, Paavonen J, Mardh PA, Stary A, Pederson BS. The accuracy and efficacy of screening tests for Chlamydia trachomatis : a systematic review. J. Med. Microbiol. 2002 51(12): 1021- 1031.