- Current Pediatric Research (2016) Volume 20, Issue 1
Prevalence of Anaemia and the Relationship between Haemoglobin Concentration and CD4 Count in HIV Positive Children on Highly Active Antiretroviral Therapy (HAART) in Lagos, Nigeria
- *Corresponding Author:
- Regina Esiovwa Ahumareze
University of the West of Scotland, Paisley, Scotland, United kingdom
E-mail: regina.esiovwa@uws.ac.uk
| Date of Acceptance | April 06, 2016 |
Abstract
Background: Anaemia is commonly reported among people living with HIV, however the prevalence of anaemia in highly active antiretroviral therapy (HAART) experienced Nigerian children has not been well defined. This study addressed this gap and evaluated the relationship between haemoglobin concentration and CD4 count in the presence of HAART.
Method: Participants (n=164) were aged between 5 and 12 years, living with HIV and had received HAART for a minimum of 12 months. All participants were outpatients at two HIV treatment centres in Lagos, Nigeria. Haemoglobin concentration and CD4 count were determined as part of baseline measurements for an ongoing randomized controlled multivitamin study (NCT02552602). Prevalence of anaemia and immune deficiency were determined based on haemoglobin concentration and CD4 count (respectively) of study participants. Pearson correlation was used to evaluate the correlation between haemoglobin concentration and CD4 count. T test was used to determine if statistical differences in haemoglobin concentration existed among participants with immune deficiency and no significant immune deficiency.
Result: At 54.2%, anaemia was still highly prevalent in HAART experienced children in Lagos, Nigeria. The prevalence of anaemia among immune deficient participants (CD4 count <500 cells/ mm3) was not significantly different from the prevalence of anaemia among participants with no significant immune deficiency (CD4 count ≥ 500 cells/mm3) (52% v 55%, Pearson Chi- Square, P= 0.783). Haemoglobin concentration was not significantly correlated with CD4 count (Pearson correlation (r) = 0.081, P = 0.302) and haemoglobin concentration could not be used as a predictor of immune status (Binary logistic regression, OR 1.461, 95% CI 0.866 – 2.464, P= 0.16).
Conclusion: Despite HAART use, anaemia is still highly prevalent among HIV positive children in Lagos, Nigeria. With the known negative influence of anaemia on HIV disease progression, it is important that measures to address anaemia in these children are evaluated and implemented.
Keywords
HIV, Anaemia, Haemoglobin, CD4 count, Nigeria.
Background
Anaemia has been reported to be one of the more common haematological disorders affecting people living with HIV (PLHIV) [1-3]. Studies have shown that the prevalence of anaemia in HIV disease can be up to 80% [4-6] depending on region and threshold used to define anaemia. HIV related anaemias may be as a result of opportunistic infections [7], as a side effect of chemotherapeutic treatment [8-11], as a result of changes in cytokine expression with resultant decrease in blood cell production [12,13], or as a consequence of micronutrient deficiencies [7].
In HIV infection, the presence of anaemia could translate to significant reduction in quality of life [14] and faster HIV disease progression [11,15]. Furthermore, anaemia is believed to be a predictor of morbidity and mortality in PLHIV [16]. The relationship between anaemia and HIV prognosis has been described in Figure 1.
Resolving anaemia in PLHIV is beneficial as a previous study reported a 170% decrease in mortality risk in PLHIV with resolved anaemia, as opposed to those with persistent anaemia [11]. Fortunately, HAART for at least 6 months is associated with resolution of anaemia [17,18], while HAART for at least 12 months is associated with protection against development of anaemia [18]. In paediatric HIV infection, declines in prevalence of anaemia in HAART settings have also been reported [19-21]. However, variations in the prevalence of anaemia reported in different parts of the world [22], reports of increased incidence of anaemia in the black race [9,11,23] as well as the relationship between malnutrition and anaemia [24,25] have made it imperative to understand the prevalence of anaemia in HAART experienced children in food insecure settings as seen in some parts of Nigeria.
A number of mechanisms through which HAART corrects prior existing anaemia have been proposed. It is believed that the ability of HAART to protect against opportunistic infections could indirectly ameliorate the negative effects of HIV infection on erythropoietin levels [26]. In addition, by inhibiting HIV-1 replication and reducing viral loads, HAART use is linked with improved hematopoietic progenitor cell growth [27,28]. Furthermore, use of protease inhibitors are also linked to a reduction in hematopoietic progenitor apoptosis [29]. Hence HAART use potentially translates to increased blood cell generation, and resolution of pre-existing anaemia.
HAART use is also associated with immune restoration which is characterised by an increase in CD4 cell count [30]. With the restoration and preservation of CD4 cells, quality of life is improved and risks of HIV related morbidity and mortality is reduced [30].
Using haemoglobin (Hb) concentration to diagnose anaemia and CD4 count to determine immune status, this study aimed to determine the prevalence of anaemia and immune deficiency among study participants. It also assessed whether a correlation existed between Hb concentration and CD4 count among study participants and if Hb concentration could be a good predictor of immune status based on CD4 count. Lastly, it evaluated if there was any difference in Hb concentration among participants with immune deficiency and those with no significant immune deficiency.
Method
Study setting and context
This study was carried out as part of baseline evaluations of participants enrolled in a multivitamin intervention study (NCT02552602). Participants were recruited from two HIV treatment centres in Lagos, Nigeria: The Nigerian Institute of Medical Research (NIMR) and the Lagos State University Teaching Hospital (LASUTH). Both hospitals provide care, treatment and support to people exposed to HIV or living with HIV. Recruitment was conducted from May 2015 to August 2015.
Study population, sample analysis and data collection
A total of one hundred and sixty four participants (n=164) were included. Inclusion criteria were; 1) Children aged 5-12 years who have tested positive to HIV; 2) Children attending the outpatient clinic at the HIV treatment centres in NIMR and LASUTH; 3) HAART use for a minimum of 12 months; 4) Children whose guardians could give informed consent. Exclusion criteria; 1) Children enrolled in other studies; 2) Children on immune suppressive therapy; 3) HAART naïve children; 4) Children with missing data for CD4 count, haemoglobin concentration and HAART initiation date.
CD4 count was used to determine immune competence and CD4 count was measured using Partec Cyflow Counter/Kits. Haemoglobin (Hb) concentration which was used to diagnose anaemia and Hb concentration was measured using Urit-12 Hb meter. Date of ART initiation was obtained from patient files.
Definitions of anaemia and immune status
Anaemia for this study was defined as severe anaemia, mild – moderate anaemia and no anaemia using Hb concentration ranging <8.0 g/dl, 8.0 g/dl – 11.4 g/dl and ≥11.5 g/dl respectively [31]. Immune status was defined as severe immune deficiency, mild- advanced immune deficiency and no significant immune deficiency using CD4 count ranging < 200 cells/mm3, 200-499 cells/mm3 and ≥ 500 cells/mm3 respectively [32].
Data analysis
A statistical software package (SPSS v22) was used for all statistical analysis. Percentages were used to describe categorical variables, as well as prevalence of immune deficiency and anaemia. Continuous variables were described using mean ± standard deviation (SD). Pearson correlation was used to determine the correlation between Hb concentration and CD4 count. Binary logistics regression was used to determine if Hb concentration could be used to predict immune status (determined by CD4 count). For binary logistic regression analysis, CD4 count was analysed as a binary categorical variable; CD4 count <500 cells/mm3 representing immune deficiency and CD4 count ≥ 500 cells/mm3 representing no significant immune deficiency. T test was used to determine if statistical difference exist in Hb concentration among participants with immune deficiency (CD4 count < 500 cells/mm3) and those with no significant immune deficiency (CD4 count ≥ 500 cells/mm3). P value of <0.05 at 95% confidence interval (CI) was used as level of statistical significance.
Ethics and Consent
This study was part of baseline evaluations for a randomized controlled multivitamin study. Ethics approvals for the multivitamin study were obtained from the Institutional Review Board of the Nigerian Institute of Medical Research, the Health Research Ethics Committee of the Lagos State University Teaching Hospital and the Ethics Committee of the School of Health, Nursing and Midwifery, University of the West of Scotland.
All participants were enrolled after written informed consent had been obtained from guardians. Written informed consent was required due to the vulnerable nature of study participants. Participant assent was also sought and obtained.
Results
Although 190 participants were enrolled into the randomized controlled multivitamin trial, data from only 164 participants were included in this study. For the 26 excluded participants, 14 participants were on HAART for less than 12 months, 10 participants were not on HAART at the time of enrolment, baseline CD4 count and Hb data for one participant was missing, while HAART initiation date for one participant was unavailable.
Of the 164 participants included in this study, 55.5% (91/164) and 44.5% (73/164) were male and female respectively. Mean (± SD) age of participants was 8.26 (± 1.91) years, while mean CD4 count (± SD) and mean Hb concentration (± SD) were 1004.39 (± 519.32) cells/ mm3 and 11.07 (± 1.41) g/dl respectively. Mean Hb concentration (± SD) in females and males were 11.13(± 1.37) g/dl and 11.02 (± 1.46) g/dl respectively, while mean CD4 count (± SD) was 1108.00 (± 566.07) cells/mm3 and 921.27 (± 465.19) cells/mm3 in females and males respectively. While there was no statistically significant difference in Hb concentration in females and males (P=0.65), females had significantly higher CD4 count compared to males (P=0.02) (Table 1).
| Variable | Value |
|---|---|
| Male | 91 (55.5%) |
| Female | 73 (44.5%) |
| Male: Female | 1.25:1 |
| Age (mean in years ± SD) | 8.26 (±1.91) years |
| CD4 count (mean ± SD) | 1004.39 (± 519.32) cells/mm3 |
| Hb concentration (mean ± SD) | 11.07 (± 1.41) g/dl |
Table 1: Participant characteristics.
The prevalence of anaemia in study participants was 54.2%. The prevalence of severe anaemia and mildmoderate anaemia was 1.2% (2/164) and 53.0% (87/164) respectively, while 45.7% (75/164) of participants were not anaemic. In 83% (137/164) of participants, there was no significant immune deficiency (CD4 count ≥ 500 cells/ mm3). However, 15.9% (26/164) and 0.6% (1/164) had mild – advanced immune deficiency (CD4 count 200-499 cells/mm3) and severe immune deficiency (CD4 count < 200 cells/mm3) respectively ) (Table 2).
| CD4 count (Based on WHO classification system [32]) | |||
|---|---|---|---|
| Measure | Group | Number (%) | Mean (±SD) |
| <200 cells/ mm3 | Severe immune deficiency | 1 (0.6) | 42 |
| 200-499cells/ mm3 | Mild – advanced immune deficiency | 26 (15.9) | 395.69 (84.981) |
| ≥500 cells/ mm3 | No significant immune deficiency | 137 (83.5) | 1126.93 (478.68) |
| Hb (Based on WHO recommendation for diagnosis of anaemia for children aged 5-11 years [31]) | |||
| <8.0 g/dl | Severe anaemia | 2 (1.2) | 6.80 (0.42) |
| 8.0 – 11.4g/dl | Mild – moderate anaemia | 87 (53.0) | 10.15 (0.92) |
| ≥11.5g/dl | No anaemia | 75 (45.7) | 12.25 (0.72) |
Table 2: Group characteristics
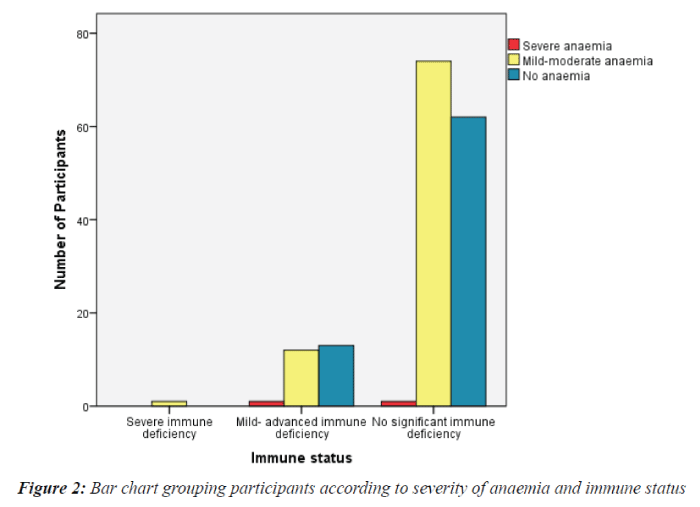
The only participant with severe immune deficiency had mild- moderate anaemia. Of the participants with mild – advanced immune deficiency, 1/26 (3.8%) had severe anaemia, 12/26(46.2%) had mild - moderate anaemia, while 13/26 (50%) did not have anaemia. Of the participants with no significant immune deficiency, 1/137 (0.7%) had severe anaemia, 74/137 (54%) had mild - moderate anaemia and 62/137 (45.3%) were not anaemic. This is described in Figure 2. For immune deficient participants (CD4 count < 500cells/mm3) and those with no significant immune deficiency (CD4 count ≥ 500 cells/mm3), 51.9% (14/27) and 54.7% (75/137) respectively were anaemic. The difference in anaemia prevalence among participants based on immune status was not statistically significant (Pearson Chi-Square, P= 0.783).
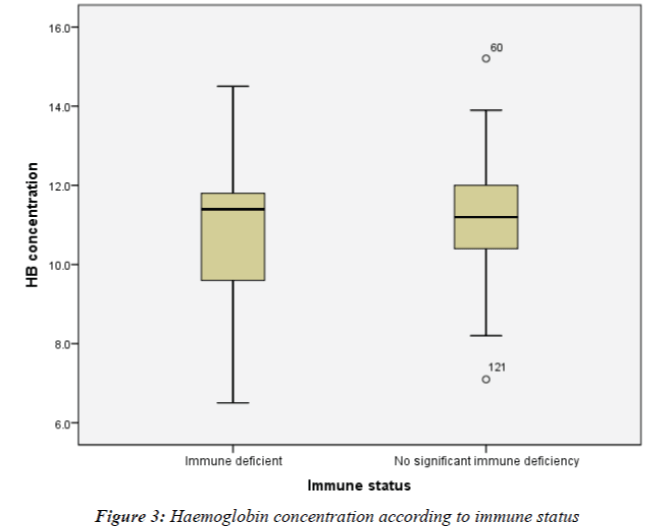
The Hb concentration for the participant with severe immune deficiency was 9.0g/dl. The mean Hb concentration (± SD) for participants in the mild-advanced immune deficiency group (CD4 count 200-499 cells/mm3) and the no significant immune deficiency group (CD4 count ≥ 500 cells/mm3) were 11.02 (± 1.80) g/dl and 11.09 (± 1.33) g/dl respectively. Due to the fact that only one participant was in the severe immune deficiency group, immune status was then evaluated as the presence or absence of immune deficiency and not as a degree of immune deficiency. Hence participants were divided into two immunological groups based on CD4 count: immune deficient (CD4 < 500 cells/ mm3) and no significant immune deficiency (CD4 ≥ 500 cells/mm3). No statistical difference in Hb concentration was observed among immune deficient participants and participants with no significant immune deficiency (10.95 ± 1.81g/dl vs11.09 ± 1.33 g/dl respectively, T-test, P = 0.63). This is described in Figure 3.
When data for all study participants (164/164) were analysed, there was no correlation between Hb concentration and CD4 count (Pearson correlation (r) = 0.081, P = 0.302). However, when data specifically for anaemic participants (89/164) were analysed, there was a weak positive correlation between Hb concentration and CD4 count (r = 0.249). Further analysis using binary logistic regression showed that Hb concentration of anaemic participants could not be used to predict immune status (OR 1.461, 95% CI 0.866 – 2.464, P= 0.16). For binary logistic regression analysis, CD4 count was analysed as a binary categorical variable; CD4 count < 500 cells/mm3 representing immune deficiency and CD4 count ≥ 500 cells/mm3 representing no significant immune deficiency ) (Table 3).
| CD4 count | Hb concentration | Total | |||
|---|---|---|---|---|---|
| <8.0 g/dl | 8.0-11.4 g/dl | ≥11.5 g/dl | |||
| <200 cells/mm3 | 0 | 1 | 0 | 1 | |
| 200-499 cells/mm3 | 1 | 12 | 13 | 26 | |
| ≥500 cells/mm3 | 1 | 74 | 62 | 137 | |
| Total | 2 | 87 | 75 | 164 | |
Table 3: Number of participants in each group based on CD4 count and Hb concentration
Discussion
Anaemia is a significant predictor of HIV disease progression [1,11,15,33,34]. Hence the need to diagnose anaemia and treat the underlying causes is pertinent to improving overall health outcomes of PLHIV. Anaemia, defined as Hb < 11.5 g/dl [31] in this study, was found to be highly prevalent (54.2%). The prevalence of severe anaemia was very low (1.2%), suggesting that severe anaemia may be a less prevailing occurrence in patients experienced on HAART [35,36]. However, mildmoderate anaemia was still highly prevalent at 53.0%. This prevalence was higher than that reported in similar studies conducted in HAART experienced children in Eastern Ethiopia (39.2%) [20], Kenya (16.6%) [19], South Western Ethiopia (21.9%) [37], 5.6% -9.2% in seven West African countries excluding Nigeria [36], as well as in Uganda and Zimbabwe (7%) [38].
Lower Hb concentration threshold used to define anaemia in some of the previous studies would have contributed to the non-comparability in reported prevalence. For some of these studies, anaemia in children was defined as Hb concentration below 10 g/dl [19,37] and 10.5 g/dl [39,40]. Hence the lower cut-offs could have considerably lowered the prevalence of anaemia and underestimated the magnitude of the problem.
Another factor that could have contributed to the high prevalence of anaemia seen amongst study participants is the possible effect of zidovudine. The known side effects of macrocytic anaemia and myelosuppression associated with zidovudine use in PLHIV have been reported in several studies [38,41-46]. With 81.1% (133/164) of study participants on zidovudine (AZT) based HAART regimen, the potential contribution of AZT to the high prevalence of anaemia may have been substantial. Other possible factors that may have contributed to the higher prevalence of anaemia in this study compared to previous studies include dissimilar methodologies used, heterogeneity of study populations, varying nutritional status of the different study populations bearing in mind the effect of malnutrition on anaemia [24,25], varying interventions used to prevent anaemia in the different settings as well as socioeconomic and geographic variations.
It is important to note that a number of studies [39,40] have also reported similarly high prevalence of anaemia in Nigerian children regardless of HIV status (49.2%; 76.9% and 57.1%; 38.6% and 57.1% respectively). Hence there is the likelihood that the high prevalence of anaemia amongst participants in this present study may be a reflection of the prevalence of anaemia in Nigerian children.
No significant difference was observed in the prevalence of anaemia among immune deficient participants (CD4 count < 500 cells/mm3) and participants with no significant immune deficiency (CD4 count ≥ 500 cells /mm3) (52% v 55%), P = 0.783. Our result contradicts previous reports of increased prevalence of anaemia in HIV positive persons with lower CD4 count [12,34,47]. It is possible that the lower CD4 count threshold used to define immune deficiency in previous studies compared to the 499 cells/ mm3 cut-off point used in this study may have contributed in part to the different results obtained. Furthermore, the inclusion of HAART naïve study participants in previous studies [12,47] as opposed to HAART experienced participants enrolled into this study may have also been partly responsible for the conflicting results. Further data analysis showed that there was no statistically significant difference in Hb concentration among immune deficient (CD4 count < 500 cells/mm3) participants and participants with no significant immune deficiency (CD4 count ≥ 500 cells/mm3) (P=0.63). This result also differs from that reported elsewhere [34]. Our findings suggest that for this group of HAART experienced participants, Hb concentration may not be associated with immune status based on CD4 count. This possibility was explored further.
Using Pearson correlation, there was no significant correlation between CD4 count and Hb concentration among participants (r = 0.081, P=0.302). This is inline with results obtained in previous studies [48-51], but inconsistent with results reported elsewhere [1,35]. A significant correlation between CD4 count and Hb concentration may possibly exist with HAART use as successful reduction of viral loads influence both CD4 count and Hb concentration. Reduced viral load results in increased CD4 count [52-54]. In addition, reduced viremia may reverse the anti-proliferative influence of cytokines thereby preventing the suppression of erythropoiesis, with consequential increase in Hb concentration [55-57]. The non-significant correlation of CD4 count and Hb concentration among this HAART experienced group made up of over 80% immune competent individuals suggests that the prevailing anaemia amongst study participants may be induced by factors unrelated to the erythropoiesis suppression effect of the virus. This therefore supports the multifactorial nature of HIV related anaemia [34,58].
When data from anaemic participants alone (89/164) were evaluated, there was a weak positive correlation between Hb concentration and CD4 count (r = 0.249). Despite this weak correlation, the potential to use Hb concentration (in anaemic patients) as a predictor for immune status (based on CD4 count) was evaluated using binary logistic regression. Results showed that after at least 12 months of HAART use, Hb concentration was not independently associated with immune status (OR 1.461, 95% CI 0.866 – 2.464, P= 0.16). Results from this present study therefore suggest that Hb concentration would be an unsuitable predictor of immune status (based on CD4 count).
More recent studies have arrived at similar conclusions supporting the non-suitability of Hb concentration for predicting CD4 count [48,50,51]. Some other studies have however reported that Hb concentration can increase the sensitivity of total lymphocyte count in predicting CD4 count [59], but this was not explored in our study.
Successful HAART use is associated with immunological recovery [60-65]. This is reflected in this study as majority of participants (137/164, 83.5%) had no significant immune deficiency. Unsurprisingly, severe immune deficiency was very uncommon in this group of HAART experienced participants (1/164, 0.6%). For the single participant with severe immune deficiency, the cause(s) of treatment failure would be identified, and treatment regimen modified accordingly.
This study is limited by the fact that (for participants with anaemia), the type of anaemia was not identified and underlying cause(s) of anaemia were not determined. Furthermore, the prevalence of anaemia in HIV negative children of comparable age and in similar settings was not determined. Hence the interpretation of our prevalence results may be limited. This study is strengthened because both male and female children were included; hence results can be generalized to similar settings in Nigeria. Furthermore, all the data used for this study were from HAART experienced children as opposed to HAART naïve children included in majority of earlier studies. This study therefore contributes to some of the newly emerging data on the prevalence of anaemia in the presence of HAART.
Despite increasing HAART access to PLHIV, this study provides an insight to a health challenge that remains unresolved in HIV positive children in Lagos, Nigeria.
Conclusion
Despite HAART use, a high prevalence of anaemia still persists among children living with HIV in Lagos, Nigeria. Furthermore the prevalence of anaemia amongst study participants appears not to be associated with CD4 count or immune status. The high prevalence of anaemia in this group of HAART experienced children may be a reflection of possibly high prevalence of anaemia amongst children in several parts of Nigeria. However, the direct effect of anaemia on HIV related morbidity and mortality requires that particular attention should be paid to HIV positive children. For these children, prompt diagnosis of anaemia, identification of the underlying cause(s) of persistent anaemia in HAART experienced patients as well as the implementation of appropriate interventions are essential.
Competing interests
The authors declare that there are no competing interests.
Authors’ contributions
REA, JR, AD, EM, ED, and OA were involved in the conceptualization and design of the study. REA, JR, AD, AW, EM, OA, YB, and AA were involved in the implementation of the study. REA, AW, YB and AA were involved in data acquisition. REA was responsible for data analysis and interpretation of data. All authors critically revised and approved the manuscript
Acknowledgements
We are thankful to the children who participated in this study and we are grateful to their guardians as well. We thank Panets Education Trust for Africa (now merged with Canon Collins Educational and Legal Assistance Trust) and the School of Health, Nursing and Midwifery, University of the West of Scotland for financial support for the study. We thank Partec Nigeria Limited for donating the CD4 count kits used for study.
The contents of this article are from the authors. The study sponsors have made no input.
References
- Obirikorang C,Yeboah F A. ‘Blood haemoglobin measurement as a predictive indicator for the progression of HIV/AIDS in resource-limited setting’. Journal of Biomedical Science 2009;16: 102.
- Buskin S E, Sullivan PS. ‘Anemia and its treatment and outcomes in persons infected with human immunodeficiency virus’.Transfusion2004;44: 826–832.
- Volberding P. ‘The Impact of Anemia on Quality of Life in Human Immunodeficiency Virus–Infected Patients’. The Journal of Infectious Diseases 2002; 185: S110–S114.
- Umar I, Hassan-Hanga F, Ibrahim M. ‘Prevalence of anaemia in paediatric patients with HIV infection in Kano’. Nigerian Journal of Paediatrics 2015; 42:103–106.
- Meidani M, Rezaei F, Maracy M, et al. Prevalence, severity, and related factors of anemia in HIV/AIDS patients’.J Res Med Sci. 2012; 17: 138–142.
- Erhabor O, Ejele O, Nwauche C et al. ‘Some haematological parameters in Human Immunodeficiency Virus (HIV) infected Africans: the Nigerian perspective’. Nigerian Journal of Medicine 2005; 14.
- Volberding PA, Levine AM, Dieterich D, et al. ‘Anemia in HIV Infection: Clinical Impact and Evidence-Based Management Strategies’.Clinical Infectious Diseases 2004; 38:1454–1463.
- Nandlal V, Moodley D, Grobler A, et al. ‘Anaemia in pregnancy is associated with advanced HIV disease’.PloS one 2014; 9.
- Wills T, Nadler J, Somboonwit C, et al. ‘Anemia prevalence and associated risk factors in a single-center ambulatory HIV clinical cohort’. AIDS Read. 2004; 14: 305–313.
- Bain BJ. ‘Pathogenesis and pathophysiology of anemia in HIV infection’. Current Opinion in Hematology 1999; 6: 89.
- Sullivan PS, Hanson DL, Chu S, et al. ‘Epidemiology of Anemia in Human Immunodeficiency Virus (HIV)-Infected Persons: Results From the Multistate Adult and Adolescent Spectrum of HIV Disease Surveillance Project’.Blood 1998; 91: 301–308.
- Munyazesa E, Emile I, Mutimura E, et al. ‘Assessment of haematological parameters in HIV-infected and uninfected Rwandan women: a cross-sectional study’. BMJ Open 2012; 2: e001600–e001600.
- Maciejewski J, Weichold F, Young N. ‘HIV-1 suppression of hematopoiesis in vitro mediated by envelope glycoprotein and TNF-alpha’. J Immunol 1994; 153: 4303–4310.
- Belperio PS,Rhew DC. ‘Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature’. The American Journal of Medicine 2004; 116:27–43.
- Mocroft A, Kirk O, Barton SE, et al. ‘Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe’. AIDS 1999; 13: 943–950.
- Anastos K, Shi Q, French A, et al. ‘Total lymphocyte count, haemoglobin, and delayed-type hypersensitivity as predictors of death and AIDS illness in HIV-1-infected women receiving highly active antiretroviral therapy’.Journal of acquired immune deficiency syndromes 2004; 35.
- Nacoulma E, Some Y, Tieno H, et al. ‘Haematological parameters evolution during the antiretroviral therapy of HIV infected patients in Burkina-Faso’. Bull SocPatholExot 2007; 100: 271–274.
- Berhane K, Karim R, Cohen MH, et al. ‘Impact of Highly Active Antiretroviral Therapy on Anemia and Relationship between Anemia and Survival in a Large Cohort of HIV-Infected Women’. JAIDS Journal of Acquired Immune Deficiency Syndromes 2004 37: 1245–1252.
- Kibaru EG, Nduati R, Wamalwa D, et al. ‘Impact of highly active antiretroviral therapy on hematological indices among HIV-1 infected children at Kenyatta national Hospital-Kenya: Retrospective study’. AIDS Res Therapy 2015; 12.
- Teklemariam Z, Mitiku H,Mesfin F. ‘Prevalence of anemia and nutritional status among HIV-positive children receiving antiretroviral therapy in Harar, eastern Ethiopa’. HIV/AIDS 2015; 5:191–196.
- Mihiretie H, Taye B, Tsegaye A. ‘Magnitude of anemia and associated factors among pediatric HIV/AIDS patients attending Zewditu memorial hospital ART Clinic, Addis Ababa, Ethiopia’. Anemia 2015.
- WHO. Worldwide prevalence of anaemia 1993–2005.WHO Global Database on Anaemia 2008.
- Levine A, Berhane K, Masri-Lavine L, et al. ‘Prevalence and correlates of anemia in a large cohort of HIV-infected women: Women’s interagency HIV study’. Journal of acquired immune deficiency syndromes 2001; 26: 28–35.
- Osazuwa F, Ayo OM. ‘Contribution of malnutrition and malaria to anemia in children in rural communities of Edo state, Nigeria’. N Am J Med Sci 2010; 2.
- Thakur N, Chandra J, Pemde H et al. ‘Anemia in severe acute malnutrition’. Nutrition 2014; 4.
- Semba RD, Shah N, Vlahov D. ‘Improvement of anemia among HIV-Infected injection drug users receiving highly active antiretroviral therapy. JAIDS 2001; 26: 315–319.
- Huang SS, Barbour JD, Deeks SG, et al. ‘Reversal of human immunodeficiency virus type 1-Associated Hematosuppression by effective Antiretroviral therapy’. Clinical Infectious Diseases 2000; 30: 504–510.
- Isgrò A, Mezzaroma I, Aiuti A, et al. ‘Recovery of hematopoietic activity in bone marrow from human immunodeficiency virus type 1-infected patients during highly active antiretroviral therapy’. AIDS research and human retroviruses 2000; 16: 1471–1479.
- Sloand E, Maciejewski J, Kumar P, et al, ‘Protease inhibitors stimulate hematopoiesis and decrease apoptosis and ICE expression in CD34(+) cells’. Blood 2000; 96: 2735–2739.
- Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of Antiretroviral agents in pediatric HIV infection 2011.
- WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity 2011.
- WHO. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance: African Region 2005.
- Tesfaye Z, Enawgaw B. ‘Prevalence of anemia before and after initiation of highly active antiretroviral therapy among HIV positive patients in northwest Ethiopia: A retrospective study’. BMC Research Notes 2014; 7: 745.
- De Santis GC, Brunetta DM, Vilar FC, et al. ‘Hematological abnormalities in HIV-infected patients’. International Journal of Infectious Diseases 2011; 15: e808–e811.
- Mata-Marín JA, Martínez-Martínez RE, Gaytán-Martínez JE, et al. ‘Risk factors and correlates for anemia in HIV treatment-naïve infected patients: A cross-sectional analytical study’. BMC Research Notes 2010; 3: 230.
- Renner L, Dicko F, Kouéta F, et al. ‘Anaemia and zidovudine-containing antiretroviral therapy in paediatric antiretroviral programmes in the IeDEA Paediatric west African database to evaluate AIDS’. Journal of the International AIDS Society 2013; 17.
- AbebeM, Alemseged F. ‘Hematologic abormalities among children on Haart, in Jimmauniversity specialized hospital, southwesternethiopia’. Ethiopian Journal of Health Sciences 2009; 19.
- Jaganath D, Walker SA, Ssali F, et al. ‘HIV-Associated anemia after 96 weeks on therapy: Determinants across age ranges in Uganda and Zimbabwe’. AIDS Res Hum Retroviruses 2014; 30: 523–530.
- Ughasoro MD, Emodi IJ, Okafor HU, et al. ‘Prevalence of moderate and severe Anaemia in children under 5 in university of Nigeria teaching hospital Enugu, southeast Nigeria’. Pediatric Research 2011; 70:489–489.
- Noland GS, Graves PM, Sallau A, et al. ‘Malaria prevalence, anemia and baseline intervention coverage prior to mass net distributions in Abia and plateau states, Nigeria’. BMC Infect Dis 2014; 14.
- Giganti MJ, Limbada M, Mwango A, et al. ‘Six-month hemoglobin concentration and its association with subsequent mortality among adults on antiretroviral therapy in Lusaka, Zambia’. J Acquir Immune DeficSyndr 2012; 61.
- Johannessen A, Naman E, Gundersen SG, et al. ‘Antiretroviral treatment reverses HIV-associated anemia in rural Tanzania’. BMC Infectious Diseases 2011; 11: 190.
- Masaisa F, Gahutu JB, Mukiibi J, et al. ‘Anemia in human immunodeficiency Virus–Infected and uninfected women in Rwanda’. Am J Trop Med Hyg 2011; 84.
- Agarwal D, Chakravarty J, Chaube L, et al. ‘High incidence of zidovudine induced anaemia in HIV infected patients in eastern India’. The Indian journal of medical research 2010; 132.
- Moyle G, Sawyer W, Law M, et al. ‘Changes in hematologic parameters and efficacy of thymidine analogue-based, highly active antiretroviral therapy: A meta-analysis of six prospective, randomized, comparative studies’. Clinical therapeutics 2004; 1.
- Koduri P, Parekh S. ‘Zidovudine-related anemia with reticulocytosis’. Annals of hematology 2003; 3.
- Shen Y, Wang Z, Lu H, et al. ‘Prevalence of anemia among adults with newly diagnosed HIV/AIDS in china’.PLoS One 2013; 8.
- Emuchay C, Okeniyi S,OkeniyiJ. ‘Correlation between total lymphocyte count, hemoglobin, hematocrit and CD4 count in HIV patients in Nigeria’.PJBS 2014; 4.
- Pande A, Bhattacharyya M, Pain S, et al. ‘Anemia in Antiretroviral Naïve HIV/AIDS Patients: A Study from Eastern India’. Online Journal of Health & Allied Sciences 2012; 10.
- Sen S, Vyas A, Sanghi S, et al. ‘Correlation of CD4+ T cell count with total lymphocyte count, haemoglobin and erythrocyte sedimentation rate levels in human immunodeficiency virus type-1 disease’. Medical Journal Armed Forces India 2011; 67:15–20.
- Alavi S, Ahmadi F,Farhadi M. ‘Correlation between total Lymphocyte count, hemoglobin, Hematocrit and CD4 count in HIV/AIDS patients’.ActaMedicaIranica 2009; 47:1–4.
- Smith C, Sabin C, Lampe F, et al. ‘The potential for CD4 cell increases in HIV-positive individuals who control viraemia with highly active antiretroviral therapy’. AIDS 2003; 7.
- Hunt P, Deeks S, Rodriguez B, et al. ‘Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy’. AIDS 2003; 13.
- Olawumi H, Olatunji P, Salami A, et al. ‘Effect of highly active antiretroviral therapy on CD4 count and weight in AIDS patients seen at the UITH, Ilorin’. Nigerian journal of clinical practice 2009; 4.
- Sarcletti M, Quirchmair G, Weiss G, et al. ‘Increase of haemoglobin levels by anti-retroviral therapy is associated with a decrease in immune activation’. European journal of haematology 2003; 1.
- Ezeonwu B, Ikefuna A, Oguonu T, et al. ‘Prevalence of hematological abnormalities and malnutrition in HIV-infected under five children in Enugu’. Nigerian journal of clinical practice 2014; 3.
- Fuchs D, Zangerle R, Artner-Dworzak E, et al. ‘Association between immune activation, changes of iron metabolism and anaemia in patients with HIV infection’. European journal of haematology 1993; 2.
- Silva E, Grotto H, Vilela M. ‘Clinical aspects and complete blood counts in children exposed to HIV-1: Comparison between infected patients and seroreverters’.Jornal de pediatria 2001; 6.
- Spacek L, Griswold M, Quinn T, et al. ‘Total lymphocyte count and hemoglobin combined in an algorithm to initiate the use of highly active antiretroviral therapy in resource-limited settings’. AIDS 2003; 9.
- WHO. Treatment and care2015.
- AIDS. Overview of HIV treatments 2015.
- Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. ‘Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial’. Lancet Infect Dis2014; 14.
- Osazuwa F, AyoOM, Imade P. ‘A significant association between intestinal helminth infection and anaemia burden in children in rural communities of Edo state, Nigeria’. N Am J Med Sci2011; 3: 30–34.
- Ekwochi U, Osuorah D, Odetunde O, et al. ‘Prevalence of iron deficiency anaemia in anaemic under-5 children in Enugu south east Nigeria’. Nigerian Journal of Paediatrics2014; 41:129.
- Wilhan EB. ‘Total lymphocyte count and hemoglobin combined to predict CD4 lymphocyte counts of less than 200 cells/mm(3) in HIV/AIDS’.ActamedicaIndonesiana2008; 40: 59–62.