Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2024) Volume 14, Issue 103
Preparation and evaluation of suppositories by Lopinavir
Yerram Varsha1*, A.V Jithan21Department of pharmaceutics, Osmania University, Hyderabad Telangana, India
2Department of Pharmaceutical Technology, Omega College of pharmacy, Ghatkasar, India
- *Corresponding Author:
- Varsha Y
Department of pharmaceutics
Osmania University
Hyderabad Telangana, India
E-mail: yerramvarsha630@gmail.com
Received: 25-Dec-2023, Manuscript No. AABPS-23-123371; Editor assigned: 28-Dec-2023, PreQC No. AABPS-23-123371 (PQ); Reviewed: 11-Jan-2024, QC No. AABPS-23- 123371; Revised: 16-Jan-2024, Manuscript No. AABPS-23-123371(R); Published: 28-Jan-2024, DOI:10.35841/aabps-14.103.211
Citation: Varsha Y, Jithan A.V. Preparation and Evaluation of Suppositories by Lopinavir. Asian J Biomed Pharm Sci. 2024;14(103):211
Keywords
Lopinavir, Croscarmellose sodium, HPMC K100M, and HPMC K200M.
Aim and Objective
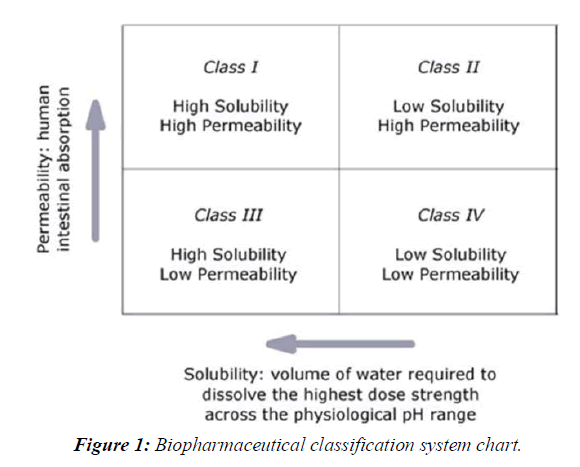
When used with ritonavir, the HIV-1 protease inhibitor lopinavir provides effective treatment for human immunodeficiency virus (HIV). Lopinavir is a BCS Class IV drug with a half-life of 6 hours.
To improve lopinavir solubility, a molding method is performed that employs the Stake 1000 and Stake 4000 base and stack starch ranges, as well as the dissolution evaporation approach employing -cyclodextrin and Croscarmellose. Made comprised the base of a suppository.
Objective
• Applying sound discretion while determining the best locations for solid-state scattering supports.
• Forlopinavir is emphasized throughout the preparation process.
• There has to be a study done comparing various pharmaceutical fillers.
• Dissolvable delivery options for broad-spectrum lopinavir efficacy: design and develop.
• Complete medical content homogeneity across a range of organized programs.
• For your improved convenience, we now offer Stake 1000 and Stake 4000 suppositories. Ingredients that do what they say they will
• Spread in vitro drug release; release energy with a decay profile.
Introduction
Oral bioavailability of a drug depends on its solubility and/or dissolution rate, and dissolution may be the rate determining step for the onset of therapeutic activity. Therefore efforts to increase drug dissolution of drug are often needed. Methods available to improve dissolution include salt formation, micronization and addition of solvent or surface active agents. Solid Dispersion (SD) is one of such methods and it involves a dispersion of one or more active ingredients in an inner carrier or matrix in solid state prepared by melting, dissolution in solvent or melting solvent method [1,2].
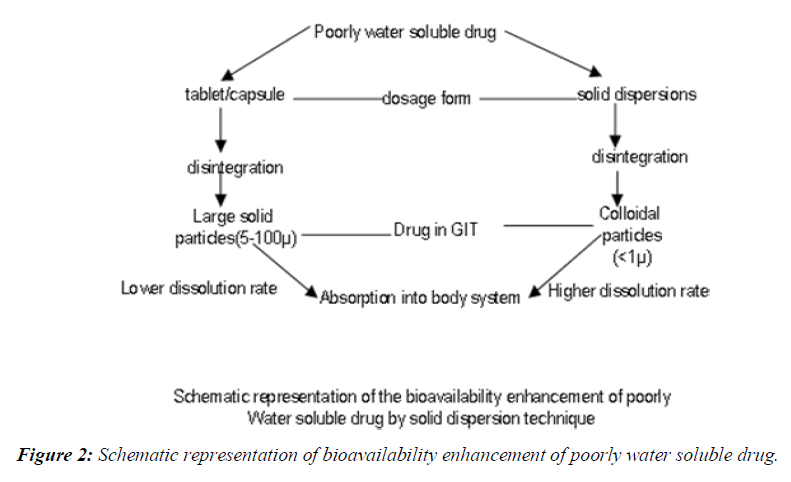
The enhancements of oral bioavailability of such poorly water-soluble drugs often show poor bioavailability because of low and erratic levels of absorption. Drugs that undergo dissolution rate limited gastrointestinal absorption generally show improved dissolution and bio availability as a result of reduction in particle size. However, micronizing of drugs often leads to aggregation and agglomeration of particles, which results in poor wettability [3, 4]. Solid dispersions of poorly water-soluble drugs with water-soluble carriers have been reduced the incidence of these problems and enhanced dissolution. The development of solid dispersions as a practically viable method to enhance bioavailability of poorly water-soluble drugs overcame the limitations of previous approaches such as salt formation, Solubilization by co-solvents, and particle size reduction. Studies revealed that drugs in solid dispersion need not necessarily exist in the micronized state [5,6]. A fraction of the drug might molecularly disperse in the matrix, thereby forming a solid dispersion. When the solid dispersion is exposed to aqueous media, the carrier dissolves and the drug releases as fine colloidal particles, (Figure 1).
Schematic representation of bioavailability enhancement of poorly water soluble drug
Oral bioavailability of a drug depends on its solubility and/or dissolution rate, and dissolution may be the rate determining step for the onset of therapeutic activity. Therefore efforts to increase drug dissolution of drug are often needed [7-9]. Methods available to improve dissolution include salt formation, micronization and addition of solvent or surface active agents. Solid dispersion (SD) is one of such methods and it involves a dispersion of one or more active ingredients in an inner carrier or matrix in solid state prepared by melting, dissolution in solvent or melting-solvent method. The technique has been used for a wide variety of poorly aqueous soluble drug. Poorly soluble drugs represent a problem for their scarce availability related to their low dissolution rate. The major drawback of low aqueous solubility is delays its absorption from the gastrointestinal tract. Solubility behavior of a drug is one of the key determinants of its oral bioavailability [10]. Noyesh-Whitney equation provides some hints as to how the dissolution rate of even very poorly soluble compounds might be improved to minimize the limitations to oral Availability, (Figure 2).

Where,
dC/dt - is the rate of dissolution,
A -is the surface area available for dissolution,
D - is the diffusion coefficient of the compound,
Cs- is the solubility of the compound in the dissolution medium,
C -is the concentration of drug in the medium at time t and
h - is the thickness of the diffusion boundary layer adjacent to the surface of the dissolving compound.
To increase the dissolution rate from equation the following approaches are available.
➢ To increases the surface area available for dissolution Decreasing the particle size of drug.
➢ Optimizing the wetting characteristics of compound surface.
➢ To decrease the boundary layer thickness.
➢ Ensure sink condition for dissolution.
➢ Improve apparent solubility of drug under physiologically relevant conditions.
➢ Drug administered in fed state is a way to improve the dissolution rate.
Of these possibilities, changes in the hydrodynamics are difficult to invoke in-vivo and the maintenance of sink conditions will depend on how permeable the gastrointestinal mucosa is to the compound as well ason the composition and volume of the luminal Fluids [11,12]. Although some research effort has been directed towards permeability enhancement using appropriate excipients, results to date have not been particularly encouraging. Administration of the drug in the fed state may be an option to improve the dissolution rate and also to increase the time available for dissolution; the likely magnitude of the food effect can be forecasted from dissolution tests in bio relevant media.
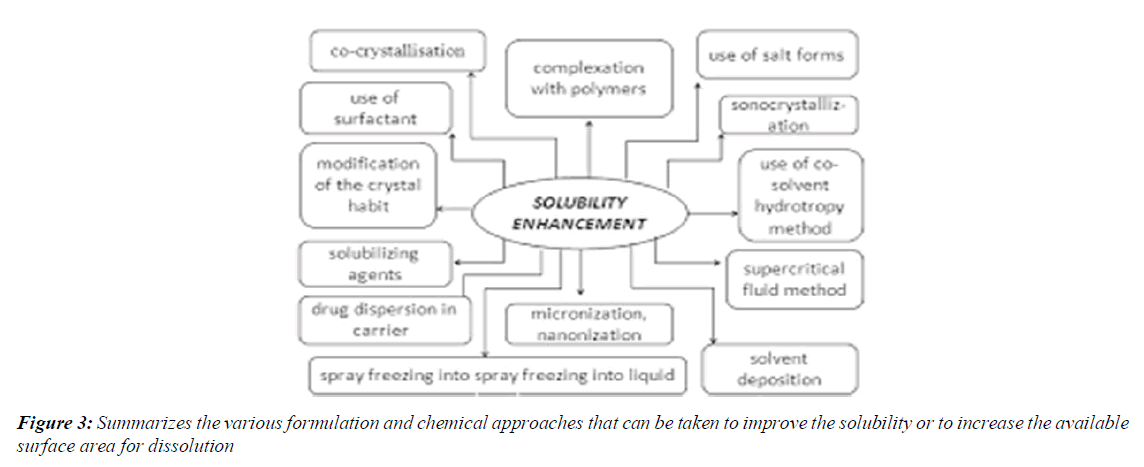
Summarizes the various formulation and chemical approaches that can be taken to improve the solubility or to increase the available surface area for dissolution
The dissolution of a drug from its solid oral dosage forms depends upon its release from the dosage form and its subsequent mixing into physiological fluids. It has been estimated that nearly 35-40% of the drugs suffer from poor aqueous solubility, thereby affecting their absorption from the gastrointestinal tract, which leads to poor oral bioavailability, high intra- and inter-subject variability, increase in dose, reduction in therapeutic efficiency and finally failure in formulation development [13-15]. The development of solid dosage forms for water-insoluble drugs has been a major challenge for pharmaceutical scientists for decades [16]. Various formulation strategies such as micronisation, micellar solubilization, complexation, dendrimers for drug solubilization, formation of solid solutions or dispersions with hydrophilic carriers, self-microemulsifying drug delivery systems, spray drying, nano approaches, pro-drug approaches and salt synthesis have been developed to increase the dissolution rate of water-insoluble drugs [17-19]. An attractive possibility is employing a simple solid dispersion technique making use of various hydrophilic carriers. Solid Dispersions (SDs) are defined as the dispersion of one or more active ingredients in an inert hydrophilic carrier or matrix in a solid state, and are prepared by the fusion, solvent or solvent-fusion method. This technique enables reducing particle size to a nearly molecular level, offers a variety of processing and excipient options that allow for flexibility when formulating oral delivery systems of poor water-soluble drugs that are cost-effective and significantly reduced in dosage [20-23]. It has been widely demonstrated that a hydrophilic carrier dissolves rapidly, exposing the drug particles to the dissolution medium as fine particles facilitating quick dissolution and absorption8. The mechanisms for increased dissolution rate may include reduction of crystallite size, solubilization effect of the carrier, absence of aggregation of drug crystallites, improved wettability and dispersability of a drug from the dispersion, dissolution of the drug in the hydrophilic carrier or conversion of the drug to an amorphous state [24,25]. Schizophrenia is a severe non-curable illness of the brain with serious consequences if not properly treated and kept under control. It is the most common form of severe mental illness, (Figure 3).
Solid dispersions
Solid Dispersions (SDs) traditionally have been used as an effective method to improve the dissolution properties and bioavailability of poorly water-soluble drugs. Since 1961, many investigators have studied SDs of poorly water- soluble drugs with various pharmacologically inert carriers to increase the dissolution and oral absorption of poorly water-soluble drug show ever, only a few systems are useful commercially [26].
Fast or immediate drug dissolution from solid dispersions has been observed due to increased wettability, improved dispersibility of drug particles, existence of the drug in amorphous form with improved solubility and absence of aggregation of drug particles .Literature shows that the solvent evaporation method has been used for the preparation of solid dispersions for dissolution enhancement .Earlier studies show that solid dispersion systems increased the drug dissolution due to improved solubility, wettability and dispersibility using hydrophilic carriers In the present work, physical mixtures, co-grinding and co-precipitation or solvent evaporation method was used to prepare solid dispersions of prednisolone [27-31]. This method requires the minimal amount of solvent in dissolving the drug. We used various polymeric carriers in this study. Polyvinylpyrrolidone (PVP) and Poly Ethylene Glycol (PEG) were chosen as water-soluble polymers.
Classification of solid dispersion
Based on their molecular arrangement, six different types of solid dispersions can be distinguished. (Table 1) Moreover, in various studies the designation of solid dispersions is based on the method of preparation [32]. However, since different preparation methods can result in the same subtypes or similar preparation methods can result in different subtypes, it can be argued that solid dispersions should preferably be designated according to their molecular arrangement. Moreover, not the preparation method but the molecular arrangement governs the properties of solid dispersions. Therefore, it is essential to use terms that indicate the molecular arrangement in the solid dispersion. Knowledge about the molecular arrangement will enlarge comprehension of the properties and behavior of solid dispersions. Furthermore, it will facilitate optimization of their properties required for a specific application [33-35]. For example, the mechanism underpinning the dissolution of solid dispersions is poorly understood. Many case studies showed accelerated dissolution of hydrophobic compounds using solid dispersions but mechanisms are rarely discussed. The most important reason for that is the lacking knowledge about the mode of incorporation of the hydrophobic drug in the matrix, despite numerous efforts to clarify this. A question like, “is the drug present as a crystalline phase or as amorphous nano-particles or molecularly dispersed throughout the matrix” is rarely discussed [36-38]. All three situations result in different drug concentrations at the dissolving interface. Still it has not been fully elucidated how this affects dissolution behaviour of solid dispersions. Secondly, the physical and chemical stability of the matrix or the incorporated drug depends on the mode of incorporation. If drug molecules, for example, are present in amorphous nano-particles, crystallization requires only rotational rearrangement. On the other hand, for a molecularly dispersed drug, translational diffusion is necessary before crystallization can occur by rotational rearrangements [39,40].
| S. No. | Properties | Value |
|---|---|---|
| 1 | Density (Bulk) | 0.529 g/cm3 |
| 2 | Density (Tapped) | 0.819 g/cm3 |
| 3 | Density (True) | 1.543 g/cm3 |
| 4 | Solubility | Insoluble in water but rapidly swells up to 4-8 times its original volume and also insoluble acetone, ethanol and toluene |
Table 1. Typical Properties of Croscarmellose Sodium.
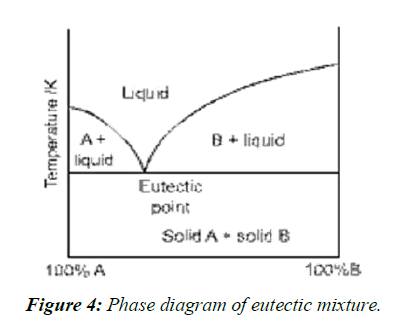
Eutectic mixtures
A simple eutectic mixture consists of two compounds which are completely miscible in the liquid state but only to a very limited extent in the solid state. It is prepared by rapid solidification of fused melt of two components that show complete liquid miscibility but negligible solid-solid solution, (Figure 4, Figure 5).
Solid solution
Solid solutions are comparable to liquid solutions, consisting of just one phase irrespective of the number of components. In the case of solid solutions, the drug's particle size has been reduced to its absolute minimum viz. the molecular dimensions and the dissolution rate is determined by the dissolution rate of the carrier. Classified according to their miscibility (continuous versus discontinuous solid solutions) or second, according to the way in which the solvate molecules are distributed in the solvendum (substitutional, interstitial or amorphous).
Continuous solid solutions
In a continuous solid solution, the components are miscible in all proportions. Theoretically, this means that the bonding strength between the two components is stronger than the bonding strength between the molecules of each of the individual components. Solid solutions of this type have not been reported in the pharmaceutical world till date.
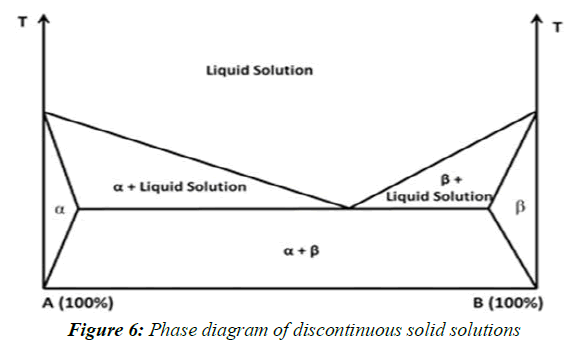
Discontinuous solid solutions
In the case of discontinuous solid solutions, the solubility of each of the components in the other component is limited. Due to practical considerations it has been suggested by Goldberg et al. That the term `solid solution' should only be applied when the mutual solubility of the two components exceeds 5% (Figure 6).
Subsitutional solid dispersions
Substitution is only possible when the size of the solute molecules differs by less than 15% or so from that of the solvent molecules. Classical solid solutions have crystalline structure, in which the solute molecules can either substitute for solvent molecules in the crystal lattice or fit into the intrsticies between the solvent molecules.
Interstitial solid solutions
In interstitial solid solutions, the dissolved molecules occupy the interstitial spaces between the solvent molecules in the crystal lattice. Solute molecule diameter should be less than 0.59 times than that of solvent molecular diameter.
Glass solution and suspensions
Glass solutions are homogeneous glassy system in which solute dissolves in glass carrier. Glass suspensions are mixture in which precipitated particles are suspended in glass solvent. Lattice energy is much lower in glass solution and suspension.
Methods of preparation of solid dispersions
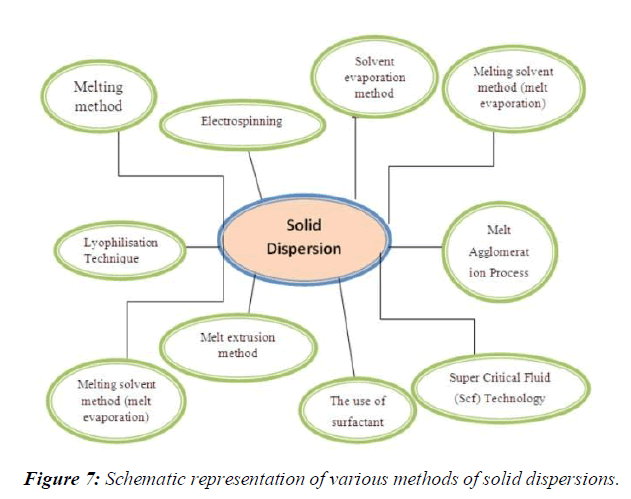
Various methods used for preparation of solid dispersion system. These methods are given bellow.
1 kneading method
2 Solvent evaporation methods
3 Melting solvent method (melt evaporation)
4 Melt extrusion methods
5 Lyophilization techniques
6 Melt agglomerations Process
7 The use of surfactant
8 Electro spinning
9 Super Critical Fluid (Scf) technology, (Figure 7)
Kneading method
In the kneading method Solid dispersions were prepared by weighed quantities of drug and polymers were placed in a mortar and then the mixtures were kneaded with small volume water for 30 min to produce a homogeneous dispersion. Once homogeneous slurry was obtained, samples where dried in oven at 450C until dryness. The dispersions after drying were pulverized using a glass mortar and pestle. The pulverized mass was then sifted through a #60 sieve to obtain a uniform particle size and stored in a desiccator at room temperature until further use.
Solvent evaporation method
In this method, the physical mixture of the drug and carrier is dissolved in a common solvent, which is evaporated until a clear, solvent free film is left. The film is further dried to constant weight. The main advantage of the solvent method is thermal decomposition of drugs or carriers can be prevented because of the relatively low temperatures required for the evaporation of organic solvents. However, some disadvantages are associated with this method such as
1) The higher cost of preparation.
2) The difficulty in completely removing liquid solvent.
3) The possible adverse effect of traces of the solvent on the chemical stability
4) The selection of a common volatile solvent.
5) The difficulty of reproducing crystal form.
6) In addition, a super saturation of the solute in the solid system cannot be attained except in a System showing highly viscous properties.
Melting solvent method (melt evaporation)
It involves preparation of solid dispersions by dissolving the drug in a suitable liquid solvent and then incorporating the solution directly into the melt of polyethylene glycol, which is then evaporated until a clear, solvent free film is left. The film is further dried to constant weight. The 5 –10% (w/w) of liquid compounds can be incorporated into polyethylene glycol 6000 without significant loss of its solid property. It is possible that the selected solvent or dissolved drug may not be miscible with the melt of the polyethylene glycol. Also the liquid solvent used may affect the polymorphic form of the drug, which precipitates as the solid dispersion. This technique possesses unique advantages of both the fusion and solvent evaporation methods. From a practical standpoint, it is only limited to drugs with a low therapeutic dose e.g. below 50 mg.
Melt extrusion method
The drug/carrier mix is typically processed with a twin-screw extruder. The drug/carrier mix is simultaneously melted, homogenized and then extruded and shaped as tablets, granules, pellets, sheets, sticks or powder. The intermediates can then be further processed into conventional tablets. An important advantage of the hot melt extrusion method is that the drug/carrier mix is only subjected to an elevated temperature for about 1 min, which enables drugs that are somewhat thermo labile to be processed. Solid dispersion by this method is composed of active ingredient and carrier, and prepare by hot-stage extrusion using a co-rotating twin-screw extruder. The concentration of drug in the dispersions is always 40% (w/w). The screw-configuration consist of two mixing zones and three transport zones distribute over the entire barrel length, the feeding rate is fix at 1 kg/h and the screw rate is set at 300 rpm. The five temperature zones are set at 100, 130, 170, 180, and 185C from feeder to die. The extrudes are collect after cooling at ambient temperature on a conveyer belt. Samples are milled for 1 min with a laboratory cutting mill and sieve to exclude particles >355μm.
Lyophilization Technique
Lyophilization involves transfer of heat and mass to and from the product under preparation. This technique was proposed as an alternative technique to solvent evaporation. Lyophilization has been thought of a molecular mixing technique where the drug and carrier are co dissolved in a common solvent, frozen and sublimed to obtain a lyophilized molecular dispersion.
Melt Agglomeration Process
This technique has been used to prepare solid dispersion wherein the binder acts as a carrier. In addition, solid dispersion are prepared either by heating binder, drug and excipient to a temperature above the melting point of the binder (melt- in procedure) or by spraying a dispersion of drug in molten binder on the heated excipient (spray-on procedure) by using a high shear mixer. The rotary processor might be preferable to the high melt agglomeration because it is easier to control the temperature and because a higher binder content can be incorporated in the agglomerates. The effect of binder type, method of manufacturing and particle size are critical parameters in preparation of solid dispersion by melt agglomeration. It has been found that the melt in procedure gives a higher dissolution rates than the spray-on procedure with PEG 3000, poloxamer 188 and gelucire 50/13 attributed to immersion mechanism of agglomerate formation and growth. In addition the melt in procedure also results in homogeneous distribution of drug in agglomerate. Larger particles results in densification of agglomerates while fine particle cause complete adhesion to the mass to bowl shortly after melting attributed to distribution and coalescence of the fine particles.
Melt Agglomeration Process by The use of surfactants
The utility of the surfactant systems in Solubilization is very important. Adsorption of surfactant on solid surface can modify their hydrophobicity, surface charge, and other key properties that govern interfacial processes such as flocculation/dispersion, floatation, wetting, Solubilization, detergency, and enhanced oil recovery and corrosion inhibition. Surfactants have also been reported to cause solvation/plasticization, manifesting in reduction of melting the active pharmaceutical ingredients, glass transition temperature and the combined glass transition temperature of solid dispersions. Because of these unique properties, surfactants have attracted the attention of investigators for preparation of solid dispersions.
Electro spinning
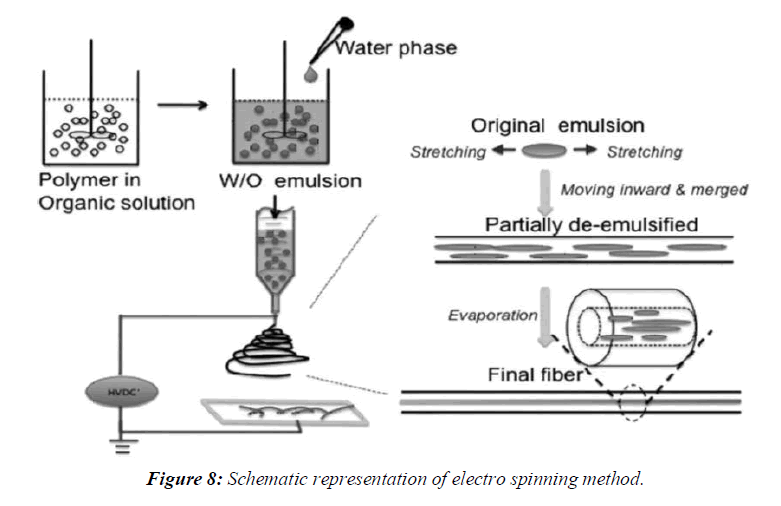
Electro spinning is a process in which solid fibers are produced from a polymeric fluid stream solution or melt delivered through a millimeter-scale nozzle. This process involves the application of a strong electrostatic field over a conductive capillary attaching to a reservoir containing a polymer solution or melt and conductive collection screen. Upon increasing the electrostatic field strength up to but not exceeding critical value, charge species accumulated on the surface of a pendant drop destabilize the hemispherical shape into a conical shape (commonly known as Taylor’s cone). Beyond the critical value, a charged polymer jet is ejected from the apex of the cone (as a way of relieving the charge built-up on the surface of the pendant drop). The ejected charged jet is then carried to the collection screen via the electrostatic force. The Columbic repulsion force is responsible for the thinning of the charged jet during its trajectory to the collection screen. The thinning down of the charged jets limited if the viscosity increases, the charged jet is dried. This technique has tremendous potential for the preparation of nano fibres and controlling the release of biomedicine, as it is simplest, the cheapest this technique can be utilized for the preparation of solid dispersions in future, (Figure 8).
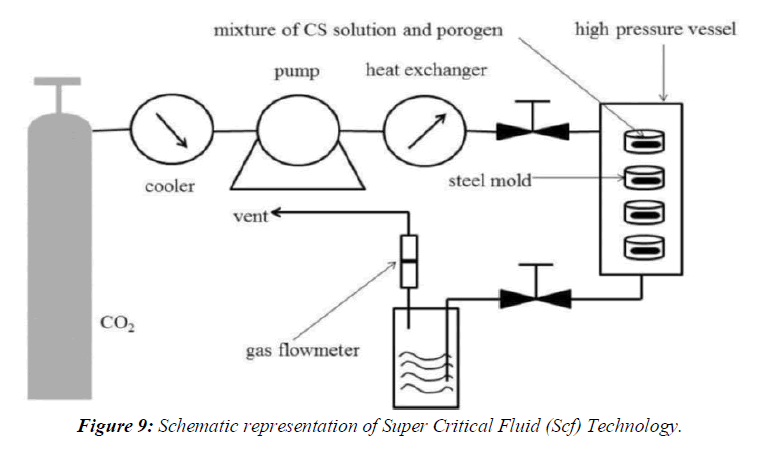
Super Critical Fluid (SCF) Technology
The supercritical fluid ant solvent techniques, carbon dioxide are used as an ant solvent for the solute but as solvent with respect to the organic solvent. Different acronyms were used by various authors to denotemicronization processes: aerosol solvent extraction system, precipitation with a compressed fluidantisolvent, gas anti-solvent, solution enhanced dispersion by supercritical fluids, and supercritical antisolvent.The SAS process involves the spraying of the solution composed of the solute and of the organic solvent into a continuous supercritical phase flowing concurrently. Use of supercritical carbon dioxide is advantageous as it is much easier to remove from the polymeric materials when the process is complete, even though a small amount of carbon dioxide remains trapped inside the polymer; it poses no danger to the patient. In addition the ability of carbon dioxide to plasticize and swell polymers can also be exploited and the process can be carried out near room temperature. Moreover, supercritical fluids are used to lower the temperature of melt dispersion process by reducing the melting temperature of dispersed active agent. The reason for this depression is the solubility of the lighter component (dense gas) in the forming phase (heavier component), (Figure 9).
Advantages of solid dispersion
Particles with Reduced Particle Size
Molecular dispersions, as solid dispersions, represent the last state on particle size reduction, and after carrier dissolution the drug is molecularly dispersed in the dissolution medium. Solid dispersions apply this principle to drug release by creating a mixture of a poorly water soluble drug and highly soluble carriers. A high surface area is formed, resulting in an increased dissolution rate and, consequently, improved bioavailability.
Particles with Improved Wettability
Carriers with surface activity, such as cholic acid and bile salts. When used, can significantly \increase the wettability property of drug. Even carriers without any surface activity, such as urea, improved drug wettability. Carriers can influence the drug dissolution profile by direct dissolution or co-solvent effects.
Particles with Higher Porosity
Particles in solid dispersions have been found to have a higher degree of porosity. The increase in porosity also depends on the carrier properties; for instance, solid dispersions containing linear polymers produce larger and more porous particles than those containing reticular polymers and, therefore, result in a higher dissolution rate. The increased porosity of solid dispersion particles also hastens the drug release profile.
Rapid disintegration of oral tablets
Drug is formulated with hydrophilic carrier (e.g. PEG) as a solid dispersion to increase its aqueous solubility and dissolution. Then superdisintegrant (e.g. croscarmellose sodium) is used in tablet formulation to achieve rapid disintegration of tablets prepared by wet granulation method. These rapidly disintegrating tablets can be used as an alternative to parenteral therapy enabling patient for self-medication even without the aid of water.
As a formulation vehicle
Solid dispersions can be used as formulation vehicle to facilitate the preclinical safety and early clinical studies on new chemical entities with very low aqueous solubility. It provides a means to rapidly assess the safety and efficacy profile of the drug substance that may be otherwise difficult to obtain.
Disadvantages of solid dispersions
The major disadvantages of solid dispersion are related to their instability. Several systems have shown changes in crystallinity and a decrease in dissolution rate with aging. The crystallization of ritonavir from the supersaturated solution in a solid dispersion system was responsible for the withdrawal of the ritonavir capsule (Norvir, Abboft) from the market.
Moisture and temperature have more of a deteriorating effect on solid dispersions than on physical mixtures. Some solid dispersion may not lend them to easy handling because of tackiness.They are not broadly used in commercial products because there is the possibility that during processing (mechanical stress) or storage (temperature and humidity stress) the amorphous state may undergo crystallization.
The effect of moisture on the storage stability of amorphous pharmaceuticals is also a significant concern, because it may increase drug mobility and promote drug crystallization.
Most of the polymers used in solid dispersions can absorb moisture, which may result in phase separation, crystal growth or conversion from the amorphous to the crystalline state or from a metastable crystalline form to a more stable structure during storage. This may result in decreased solubility and dissolution rate. Therefore, exploitation of the full potential of amorphous solids requires their stabilization in solid state, as well as during in vivo performance.
• Poor scale-up for the purposes of manufacturing.
• Laborious and expensive methods of preparation.
• Reproducibility of physicochemical characteristics.
• Difficulty in incorporating into formulation of dosage forms.
• Scale-up of manufacturing process.
• Stability of the drug and vehicle.
Applications of Solid Dispersions
Solid dispersion systems can provide numerous additional benefits; some of them are as follows
In improving immunosuppressive therapy in lung transplant patients, dry powder formulation consisting of a solid dispersion (e.g. Cyclosporine A) for inhalation is prepared. It can avoid many problems like use of local anaesthesia and irritating solvents, Solid dispersion formulations were demonstrated to accelerate the onset of action for drugs such as nonsteroidal antiinflammatory drugs (NSAIDS) where immediacy of action is crucial in relieving acute pain and inflammation.Solid dispersion systems were shown to provide bio available oral dosage forms for anti-cancer drugs, which could be substituted for standard injections to improve patient comfort and compliance. Solid dispersion systems were also found to reduce food effect on drug absorption, thus increasing the convenience of drug therapy as the need for some drugs to be taken with food was eliminated. Solid dispersion- based dosage form allowed for greater drug loading per dose and improved stability over a soft gelatin capsule formulation which thereby improved the convenience of drug therapy by reducing the dosing regime and eliminating the need for refrigerated storage. Improved absorption efficiency demonstrated for solid dispersion systems allows for a reduction in the content of active agent per dose, thus decreasing the cost associated with these drug therapies [40-43].
It also act as a functional carriers that offer the added benefit of targeting the release of highly soluble forms of poorly water soluble drugs to an optimum site for absorption. These benefits demonstrate the current contributions and future potential of solid dispersion systems toward improving drug therapies for a variety of important medical conditions whose treatment involves poorly water soluble drugs.
• To obtain a homogeneous distribution of a small amount of drug in solid state.
• To stabilize the unstable drug.
• To dispense liquid (up to 10%) or gaseous compounds in a solid dosage.
• To formulate a fast release primary dose in a sustained released dosage form.
• To formulate sustained release regimen of soluble drugs by using poorly soluble or insoluble carriers.
• To reduce pre systemic inactivation of drugs like morphine and progesterone.
• Polymorphs in a given system can be converted into isomorphous, solid solution, eutectic or molecular addition compounds.
• To increase the solubility of poorly soluble drugs thereby enhance the dissolution rate, absorption and bioavailability.
• To obtain a homogeneous distribution of a small amount of drug in solid state.
• To stabilize unstable drugs and protect against decomposition by processes such as hydrolysis, oxidation, racemization, photo oxidation etc.
• To dispense liquid or gaseous compounds;
• To formulate a fast release priming dose in a sustained release dosage form;
• To formulate sustained release preparation of soluble drugs by dispersing the drug in poorly soluble or insoluble carrier;
• To reduce side effects-(a) the binding ability of drugs for example to the erythrocyte membrane is decreased by making its inclusion complex, (b) the damage to the stomach mucous membranes by certain non-steroidal anti-inflammatory drugs can be reduced by administration as an inclusion compound;
• To mask unpleasant taste and smell and avoid undesirable incompatibilities.
• To convert liquid compounds into formulations. Liquid drugs can be manufactured as solid drug formulations such as powders, capsules or tablets e.g., unsaturated fatty acids, essential oils, nitroglycerin, benzaldehyde, prostaglandin, clofibrate etc.
• To reduce pre systemic inactivation of drugs like morphine and progesterone
Limitations
The limitations of this technology have been a drawback for the commercialization of solid dispersions. The limitations include
• Laborious and expensive methods of preparation,
• Reproducibility of physicochemical characteristics,
• Difficulty in incorporating into formulation of dosage forms,
• Scale-up of manufacturing process, and
• Stability of the drug and vehicle.
Suppositories
Suppositories are a dosage form designed to deliver drugs through rectal and vaginal routes of administration. They evolved as a more convenient alternative form of drug delivery from liquid enema formulations. In fact, the term suppositories come from the Latin word supponere, meaning ‘substitute’ [44]. While commonly perceived to be for rectal administration only, suppositories are also appropriate for vaginal administration. Pessaries are often used to describe vaginal suppositories. The Latin term pessarium derived from the Greek word pesos, which means ‘oval stone’ was used to describe the shape.
Suppositories and pessaries as drug delivery vehicles are not new dosage forms. Rectal drug delivery is one of the world's oldest strategies for drug dosing and rectally applied products have existed for hundreds of years. Suppositories have been referenced in the Hebrew Scriptures. Even vaginally applied pessaries are equally as old with documentation reported in Egyptian papyruses. Hippocrates wrote of various acorn-based medicines delivered rectally and vaginally for local pharmacologic effects [45].
Initially, rectal suppositories were composed of bases of baked honey, soap, tallow or horn impregnated with medicinal substances. In the late 18th century, cocoa butter was substituted as the primary base. The first record of the inclusion of an active pharmaceutical ingredient (API) in a suppository was in 1841 with the introduction of opium to cocoa butter. Suppositories during this time were approximately 5 g in size. In 1897, a combination of gelatin, glycerin and water was utilized for the first time. Fatty bases (triglycerides) were introduced as suppository bases during World War II due to shortages in cocoa butter. Following World War II, most commercial suppositories continued to be comprised of these hard fats as their primary bases. Also, during this time, the size of the suppositories was reduced to ∼2 g. Today, suppositories on the commercial market continue to adhere to these general product characteristics. The large majority of suppositories on the market are utilized for topical relief (paracetamol and caffeine 46) or for incontinence (bisacodyl, lactulose and glycerin 46). However, the US FDA lists several other drug products which are approved for rectal administration to treat ulcerative colitis (mesalamine, hydrocortisone) 46 and as an antipsychotic drug (prochlorperazine) [46]. For vaginal suppositories, the FDA lists compounds to treat vaginal yeast infections and bacterial vaginosis (clotrimazole, itraconazole) 47 and for hormone replacement therapies (progesterone) [47]. In Europe and Japan, several other drugs have been approved for rectal administration including products for epilepsy (diazepam) and pain (ibuprofen). In summary, the primary current approved uses of suppositories are laxative, analgesic, anti-inflammatory and antiemetic [48].
However, despite its age and current use today, the definition of a suppository is remarkably nonspecific. From the US, European and Japanese Pharmacopoeias (USP, EP and JP, respectively), only the EP has a specific chapter on rectal dosage forms [49]. The USP and JP only define a suppository as a dosage form adapted for application into the rectum [50,51]. And while more commonly used rectally, the JP does include vaginal administration into the monograph which defines a suppository. Thus suppositories are defined as a route of administration characterized by administration into the rectum or vagina to provide local or systemic effect. Yet, in this open formulation space for suppository design, suppositories still primarily follow traditional designs and criteria rather than developing systematic and rationally designed formulations. Therefore, it is under these open guidelines that the development of suppositories into viable antiviral drug delivery dosage forms is being conducted today.
The suppository dosage form
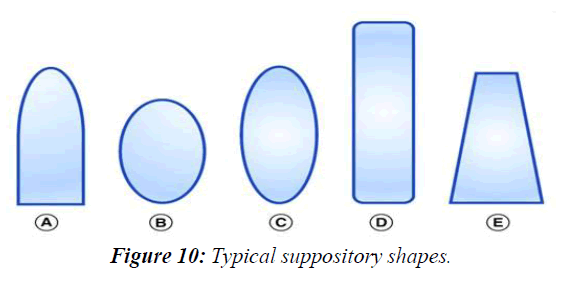
Suppositories have classically been cylindrical in geometry, longer than wide, with the most common shape being the ‘bullet’ or ‘torpedo’ shape. However, other commonly used shapes for suppositories include round and elongated ovals, tampon and ‘teardrop’ or ‘cone’ (Figure 1). These suppositories can be composed of, but not limited to, cocoa butter, coconut oil, glycerinated gelatin, hydrogenated vegetable oils and hard fats, polyethylene Glycols (PEGs) and fatty acid esters of PEG. With a combination of these excipient bases, suppositories have fallen into one of two major types: lipophilic based or hydrophilic based. The lipophilic fat-based suppositories melt at body temperature to release drug to the body. They readily solubilize typical insoluble small-molecule drugs and require no localized fluids to spread and release drug. Typically, such suppositories are ideal for the rectum where there is little available fluid in the lower large intestinal tract. The hydrophilic water-based suppositories are unaffected by body temperature and require water to dissolve the suppository and release the drug. In contrast to the fat-based suppositories, hydrophilic suppositories can more easily support drug delivery of soluble drug compounds and use the body fluids to dissolve the suppository to transport the drug into the body. Such water-based suppositories are appropriate for vaginal application where there is more local fluid. Therefore, these two different types of suppositories have allowed this dosage form to become increasingly specific for drug delivery, (Figure 10).
In the field of HIV-prevention strategies, despite the implementation of the oral pre-exposure prophylaxis Truvada®, topical vaginal and rectal microbicides remain a significant arm in the Antiretroviral Therapy (ART)-based prevention pipeline. These topical delivery vehicles are being developed as strategies to provide additional affordable and effective options for at-risk populations. However, when considering topical drug delivery for both vaginal and rectal administration, the environment and population in which the product will act must be considered when choosing an appropriate dosage form. Therefore, a ‘one size fits all’ topical HIV prevention dosage form may not be possible. Several formulations may be necessary to accommodate the regional preferences and acceptability of end users. In addition to acknowledging user preferences, a successful topical ART requires the systematic integration of drug candidates with excipients in a scientifically rational way in order to produce a safe and stable product which serves to deliver the drug candidate efficiently to the appropriate target site at the appropriate and effective concentration. From the pre formulation development, the appropriate dosage form is dependent upon factors such as the physicochemical characteristics and antiviral mechanism of action of the drug product under development.
Currently, there are several strategies being developed and investigated for vaginal and rectal topical antiviral drug delivery 52-60. Semisolid dosage forms, or topical gels, are the most common products developed for vaginal and rectal delivery and several of these semisolid products have progressed to late phase clinical development, including tenofovir (TFV) gel 61, dapivirine (DPV) gel 60 and a dual vaginal and rectal IQP-0528 containing DuoGel 58-62. Despite wide-spread use and the completion of many preclinical and clinical evaluations of gel formulations, the semisolid nature of the products result in leakage or general ‘messiness’ being a common problem experienced. Recently in the HIV microbicide VOICE trial 63, it was reported that while the efficacy of the TFV gel was high, adherence to gel use was significantly low, rendering the overall effectiveness of the gel product <40% 64-66. Because of the low adherence rate, further development of semisolid gels as a topical antiviral drug delivery formulation has dramatically been deprioritized by funding organizations such as the NIH and the Bill and Melinda Gates Foundation. As such, solid dosage forms, including intravaginal rings and quick dissolving films, have been proposed as replacement formulations and continue to see development 67.68. However, rings and films are not dosage forms that are an appropriate fit for rectal drug delivery.
Current suppository formulation strategies for antiviral drug delivery
Despite the long history of suppository use for both vaginal and rectal drug delivery, the development of suppositories for antiviral drug delivery is in its infancy. Numerous studies, particularly in the development of anti-HIV drugs, demonstrate the potential for both vaginal and rectal drug delivery to prevent and treat HIV infections 69. So, it is not surprising that it is primarily a lack of user perception and acceptability rather than pharmaceutical feasibility that has limited wide spread development of the suppository as a drug delivery vehicle.
Plan of work
Pre formulation studies
• Solubility
• Spectrophotometric studies
• Compatibility studies
Formulation
Preparation of Lopinavir Solid Dispersions loaded Suppositories.
Evaluation studies
• Estimation of drug content
• In- vitro dissolution studies
• Hardness,
• Melting point,
• Disintegration time and
• Drug content of suppository
• Dissolution studies
• Release kinetics
Review of literature
Herein, we evaluate the potential of using a simple solvent granulation process to prepare a binary drug amorphous solid dispersion (ASD) containing two anti-HIV drugs, ritonavir and lopinavir. The drugs were granulated onto a mixture of lactose and microcrystalline cellulose, followed by drying to remove the solvent. The resultant granules were characterized and each drug was found to be X-ray amorphous. No crystallization was observed following storage for 1 month under accelerated stability conditions (40 °C and 75% relative humidity). The dissolution behavior of the compacted granules was compared with the marketed formulation. The dissolution rate of ritonavir was found to be significantly retarded relative to the commercial product when the two drugs were co-granulated. However, comparable release could be achieved when each drug was individually granulated, followed by combination and compaction. The solvent granulation approach may be a viable method to make ASDs of low dose drugs with low crystallization tendencies, As a biopharmaceutical classification system Class IV drug,lopinavir(LPV) shows relatively poor water solubility and permeation in vivo. In the study, we developed novel solid dispersions (SD) of LPV to improve its bioavailability and to describe their overall behaviors. By employing solvent evaporation for a preliminary formulation screening, the SDs of LPV-polymer-sorbitan monolaurate (SBM, as the wetting agent) at 1:4:0.4 (w/w) dramatically enhanced the LPV dissolution in a non-sink medium, and then hot-melt extrusion (HME) was applied to improve the dissolution further. A hydrophilic polymer -KollidonVA 64 (VA64) and a polymeric surfactant Soluplus were employed as matrix respectively in the optimized formulations. The dissolution profiles of extrudates were significantly higher than those of SDs prepared with solvent-evaporation method. It was attributed to the stronger intermolecular interactions between LPV and the polymers in the HME process, which was also supported by the stability analysis after 6 months storage under 25 °C/60% RH. Thedifferential scanning calorimetric,Fourierand equilibrium studies showed VA64 only created hydrogen bonding (H-bond) with LPV, but Surplus generated both H-bond andmicellethanks to its amphiphilic structure. In addition, the bioavailability of LPV in Surplus matrixed extrudate was 1.70-fold of VA64 matrixed extradite and 3.70-fold of LPV crystal. In situ permeability and Caco-2 cell transport studies revealed that Soluplus significantly enhanced the permeability of LPV through rat intestine and Caco-2 cell monolayers by P-glycoprotein (P-gp) inhibition. Herein, Soluplus matrixed extradite improved the LPV bioavailability through three mechanisms: H-bond with LPV,micelleformation in water and P-gp inhibition in vivo. These unique advantages of Soluplus suggested it is a promising carrier for poorly water soluble drugs, especially the substrates of P-gp.
Amorphous Solid Dispersions (ASDs), where the drug is dispersed in a polymer, have become increasingly prevalent as a formulation strategy for the oral delivery of poorly soluble drugs due to their potential for substantial solubility enhancement. However, ASDs are susceptible to amorphous-amorphous phase separation, which may promote crystallization and/or alter the release performance. Nevertheless, the mechanisms by which phase separation and subtle microstructural changes affect ASD release remain poorly understood. Therefore, understanding the microstructure of ASDs and the subsequent implication for ASD performance are critical to design an optimally performing formulation. In this study, comprehensive investigations of microstructure evolution in lopinavir ASDs, prepared using a solvent-based process, were undertaken. Atomic force microscopy (AFM)-based nanoscale thermal analysis (nanoTA) enabled characterization of local composition at the submicron scale. The formation of heterogeneous domains was found to improve the in vitro release of lopinavir from lopinavir–hydroxypropylmethylcellulose (HPMC) ASDs for drug loadings above 33% w/w. The composition and amount of each phase formed, as well as the size and location of drug-rich phases, were found to be critical factors contributing to the altered release kinetics observed. This study highlights the complexity and importance of ASD microstructure and should contribute to a broader understanding of ASD release mechanisms.
B-Cyclodextrin complexes of meloxicam were prepared by solvent evaporation technique in different ratios to enhance the solubility of the drug. The complex was characterized by infrared spectroscopy and differential scanning calorimetric studies. There was no interaction between drug and carrier. Based on physical characters and in vitro drug release pattern, 1:3 drug-carrier ratio was selected as ideal batch for suppositories. A water-soluble base, polyethylene glycol, was selected as ideal base for the preparation of suppositories. The suppositories were prepared by moulding technique. The ideal batch of solid dispersion was incorporated into suppository base. The prepared suppositories were characterized for hardness, melting point, disintegration time and drug content. All these properties were found to be ideal. The in vitro drug release pat- tern was determined by rotating dialysis bag method. The in vitro release of meloxicam from its solid dispersion incorporated suppositories was significantly improved when compared to the intact bulk drug incorporated suppositories.
The potential of Solid Dispersion (SD) technique to improve the dissolution of haloperidol (Hal) and to develop Hal rectal suppository was investigated. Hal solid dispersions with hydrophilic carriers, namely, polyethylene glycol 6000 (PEG 6000) or sodium Starch Glycolate (SSG) in different mixing ratios were prepared by kneading technique. Dissolution studies in phosphate buffer pH 7.4 using USP paddle method were performed for Hal and its physical mixtures and solid dispersions. Fourier- Transform Infrared Spectroscopy (FTIR), Differential Scanning Calorimetry (DSC), and X-ray Powder Diffraction (XRD) analysis were performed to identify the physicochemical interactions between the drug and carrier, hence its effect on dissolution. Hal suppositories were prepared using water soluble base, polyethylene glycol (PEG 6000, 40% w/w and PEG 400, 60% w/w) and oleaginous base (Witepsol H15) utilizing molding technique. The prepared suppositories were tested for hardness, melting time, weight variation and drug content. All these properties were found to be satisfactory for practical use. The dissolution of Hal was improved significantly from its kneaded products with both carriers. Highest dissolution rate of the drug was obtained from Hal solid dispersions at the mixing ratio of 1:7 drug: carriers ratios. SSG was superior in dissolution enhancement of Hal from its solid dispersion. FTIR spectra suggested the presence of hydrogen bonds between the carriers hydroxyl groups and the drug. Powder-XRD technique in combination with differential scanning calorimetry revealed that Hal existed in crystalline form in PEG and SSG polymers. Drug release from the water soluble suppository base was greater than that from oleaginous base. Maximum drug release was obtained from suppositories containing water soluble base incorporated with Hal solid dispersion.
The aim of this work was to develop the best formulations for naproxen suppositories. The effects of different bases and surfactants on the physicochemical characteristics of the suppositories were determined by several tests such as weight variation, melting point, assay, hardness, and release rate. All formulations met the standard criteria for tested physicochemical parameters; weight variation (97-112%), content uniformity (97-105%), melting point (4.66-8.7 min) and hardness tests (>5400 g). Based on release rate studies, hydrophilic, and lipophilic bases without surfactants were not suitable bases for naproxen suppository. Amongst the formulations containing surfactants only Witepsol H15 with 0.5% w/w of Tween 80 and Witepsol W35 with 0.5% of cetylpyridinium chloride were suitable and released nearly complete drug during 30 and 60 min, respectively. This study demonstrates the effects of incorporation of known agents on the in vitro release characteristics of naproxen suppository.
Lornoxicam suppositories were prepared by using water soluble and oil soluble suppository bases. All the prepared suppositories were evaluated for various physical parameters like weight variation, drug content, hardness, Liqification time and temperature, disintegration and macro-melting range. In-vitro release study was performed USP type I apparatus (Basket type) using phosphate buffer pH 7.4 as dissolution media. The suppositories prepared were within permissible range of all physical parameters. In vitro drug released from water soluble bases (like PEG) was greater than that from oil soluble bases. Addition of HPMC, Glyceryl Behenate in agar suppositories to controlled release. The results suggest that of PEG of low molecular weight with high molecular weight in different percentage of release. The Sustained release suppositories can be prepared by addition of HPMC, Glyceryl Behenate in agar based suppositories and by use of bees wax in cocoa butter as base.
Intrauterine adhesions cause several gynecological problems. Althaea officinalis L. roots known as marshmallows contain polysaccharides (M.P.) which possess anti-inflammatory and antiulcerogenic activities also can form a bio-adhesive layer on damaged epithelial membranes prompting healing processes. Vaginal formulations of herbal origin are commonly applied to relieve cervico-uterine inflammation. Herein, we aim to develop and evaluate vaginal suppositories containing polysaccharides isolated from the A. officinalis root. Six formulations (four P.E.G.-based and two lipid-based suppositories containing 25% and 50% M.P.) met standard requirements, which were then subjected to qualitative and quantitative evaluation. All suppositories exhibited acceptable weights, hardness, content uniformity, melting point, and disintegration time, which fall within the acceptable recommended limits. Higher concentrations of M.P. in PEG-bases moderately increased the hardness (p<0.05). PEG-formulations showed content uniformity>90% of the average content while it was 75-83% for suppocire formulations. All formulations disintegrated in<30minutes. In-vitro release test revealed that M.P. release from 25%-MP formulations was higher than that of 50%-M.P. suppositories. Overall, results revealed the feasibility of preparing PEG.- or lipid-based suppositories containing M.P., which met the B.P. quality requirement.
The aim of the present study was to formulate chlortenoxicam into rectal suppositories as a new dosage form, to avoid its reported gastric irritation and to provide a rapid onset of action for children. Suppositories were prepared using fatty bases mixtures of poly (ethylene glycol), PEGs, with different molecular weights. The prepared suppositories were investigated for their weight variation, drug content, melting point, fracture point, disintegration time and in-vitro release pattern. The in vitro release study was performed USP type-I apparatus (basket type) using phosphate buffer pH7.4 as dissolution media. The suppositories prepared were within permissible range of all physical parameters, in -vitro drug release from water soluble bases like PEG was greater than that of oil soluble bases. Addition of HPMC in agar suppositories to control release. The result suggests that of PEG of low molecular weight with high molecular weight in different percentage of release. The sustained release suppository can be prepared by addition of HPMC in agar based suppositories and by use of beeswax in cocoa butter as base.
Ketotifen KT is one of antiallergic drugs, due to its first pass effect, the bioavailability of the drug is only 50%. The objective of this study was to formulate and evaluate suppositories containing KT and/or KT solid dispersion. The in-vitro release of KT from suppositories was done using dialysis membrane method in phosphate buffer at pH 7.4. The release of KT from water soluble suppository bases was higher than that from fatty or emulsion suppositories bases. Among all PEG
References
- Noyes, A.A., and Whitney W.R., (1897). The rate of solution of solid substances in their own solutions. J Am Chem Soc. 19: 930-934.
- Van Drooge, D.J. et al. (2006). Characterization of the molecular distribution of drugs in glassy solid dispersions at the nano-meter scale, using differential scanning calorimetry and gravimetric water vapour sorption techniques. Int J Pharm. 310: 220–229.
- Galia E, Nicolaides E, Hörter D, et al. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharmace Res. 1998;15:698-705.
- Kumar SG, Mishra DN. Preparation and evaluation of solid dispersion of meloxicam with skimmed milk. Yakugaku Zasshi. 2006;126(2):93-7.
- Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharmace Sci. 1997;86(1):1-2.
- Hörter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Advanc Drug Delive Rew. 2001;46(1-3):75-87.
- Taylor LS, Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharmace Res. 1997;14:1691-8.
- Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharmace Sci. 1971;60(9):1281-302.
- Goldberg AH, Gibaldi M, Kanig JL. Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures I: Theoretical considerations and discussion of the literature. J Pharmace Sci. 1965;54(8):1145-8.
- Herzfeldt CD, Kreuter J, editors. Grundlagen der Arzneiformenlehre: Galenik 2. 2013.
- Chiou WL, Riegelman S. Preparation and dissolution characteristics of several fast-release solid dispersions of griseofulvin. J Pharmace Sci. 1969;58(12):1505-10.
- Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. 2007;12(23-24):1068-75.
- Sekiguchi K, Obi N, Ueda Y. Studies on Absorption of Eutectic Mixture. II. Absorption of fused Conglomerates of Chloramphenicol and Urea in Rabbits. Chem Pharmace Bulle. 1964;12(2):134-44.
- Levy G. Effect of particle size on dissolution and gastrointestinal absorption rates of pharmaceuticals. Amer J Pharm Sci Suppor Public Health. 1963;135:78-92.
- Kanig JL. Properties of fused mannitol in compressed tablets. J Pharmac Sci. 1964;53(2):188-92.
- Goldberg AH, Gibaldi M, Kanig JL. Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures I: Theoretical considerations and discussion of the literature. J Pharmace Sci. 1965;54(8):1145-8.
- Simonelli AP, Mehta SC, Higuchi WI. Dissolution rates of high energy polyvinylpyrrolidone (PVP)-sulfathiazole coprecipitates. J Pharmac Sci. 1969;58(5):538-49.
- Chiou WL, Riegelman S. Preparation and dissolution characteristics of several fast-release solid dispersions of griseofulvin. J Pharmac Sci. 1969;58(12):1505-10.
- Urbanetz NA. Stabilization of solid dispersions of nimodipine and polyethylene glycol 2000. Eur J Pharma Sci. 2006;28(1-2):67-76.
- Vilhelmsen T, Eliasen H, Schæfer T. Effect of a melt agglomeration process on agglomerates containing solid dispersions. Inter J Pharma. 2005;303(1-2):132-42.
- Goldberg AH, Gibaldi M, Kanig JL. Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures II: experimental evaluation of a eutectic mixture: urea-acetaminophen system. J Pharma Sci. 1966;55(5):482-7.
- Hitzer P, Bäuerle T, Drieschner T, et al. Process analytical techniques for hot-melt extrusion and their application to amorphous solid dispersions. Anal Bioanalyt Chem. 2017;409:4321-33.
- Breitenbach J. Melt extrusion: From process to drug delivery technology. Euro J Pharma Biopharmac. 2002;54(2):107-17.
- Chokshi R, Zia H. Hot-melt extrusion technique: A review. Iran J Pharma Sci. 2004;3(1):3-16.
- Perissutti B, Newton JM, Podczeck F, et al. Preparation of extruded carbamazepine and PEG 4000 as a potential rapid release dosage form. Euro J Pharma Biopharmac. 2002;53(1):125-32.
- Vilhelmsen T, Eliasen H, Schæfer T. Effect of a melt agglomeration process on agglomerates containing solid dispersions. Inter J Pharma Sci. 2005;303(1-2):132-42.
- Tsinontides SC, Rajniak P, Pham D, et al. Freeze drying principles and practice for successful scale-up to manufacturing. Inter J Pharma. 2004;280(1-2):1-6.
- Seo A, Schæfer T. Melt agglomeration with polyethylene glycol beads at a low impeller speed in a high shear mixer. Euro J Pharma Biopharmac. 2001;52(3):315-25.
- Allen L, Ansel HC. Ansel's pharmaceutical dosage forms and drug delivery systems. Lippin Williams Wilkins; 2013.
- Modi A, Tayade P. Enhancement of dissolution profile by solid dispersion kneading technique. AAPS pharmscitech. 2006;7:E87-92.
- Jachowicz RE, Nürnberg E, Pieszczek B, et al. Solid dispersion of ketoprofen in pellets. Inter J Pharma. 2000;206(1-2):13-21.
- Akiladevi D, Shanmugapandiyan P, Jebasingh D, et al. Preparation and evaluation of paracetamol by solid dispersion technique. Int J Pharm Pharm Sci. 2011;3(1):188-91.
- Habib MJ. Pharmaceutical solid dispersion technology. CRC Press; 2000.
- Craig DQ. The mechanisms of drug release from solid dispersions in water soluble polymers. Inter J Pharma. 2002;231(2):131-44.
- Sethia S, Squillante III E. Solid dispersions: revival with greater possibilities and applications in oral drug delivery. Critical Rev Therap Drug Carrier Syst. 2003;20(2&3).
- Shah SM, Sultan AH, Thakar R. The history and evolution of pessaries for pelvic organ prolapse. Intern Urogyneco J. 2006;17:170-5.
- Jannin V, Lemagnen G, Gueroult P, et al. Rectal route in the 21st Century to treat children. Adva Drug Deliv Rev. 2014;73:34-49.
- Ham AS, Ugaonkar SR, Shi L, et al. Development of a combination microbicide gel formulation containing IQP-0528 and tenofovir for the prevention of HIV infection. J Pharmace Sci. 2012;101(4):1423-35.
- Dezzutti CS, Rohan LC, Wang L, et al. Reformulated tenofovir gel for use as a dual compartment microbicide. J Antimicr Chemoth. 2012;67(9):2139-42.
- Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One. 2010;5(2):e9310.
- Akil A, Parniak MA, Dezzutti CS, et al. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Translati Res. 2011;1:209-22.
- Gupta KM, Pearce SM, Poursaid AE, et al. Polyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1. J Pharmac Sci. 2008;97(10):4228-39.
- Dezzutti CS, Yandura S, Wang L, et al. Pharmacodynamic activity of dapivirine and maraviroc single entity and combination topical gels for HIV-1 prevention. Pharmace Res. 2015;32:3768-81.
- Das Neves J, Martins JP, Sarmento B. Will dapivirine redeem the promises of anti-HIV microbicides? Overview of product design and clinical testing. Adva Drug Delive Rev. 2016;103:20-32.
- Naicker N, Naidoo A, Werner L, et al. Efficacy and safety of tenofovir-containing antiretroviral therapy in women who acquired HIV while enrolled in tenofovir gel prophylaxis trials. Anti Thera. 2017;22(4):287-93.
- Pereira LE, Mesquita PM, Ham A, et al. Pharmacokinetic and pharmacodynamic evaluation following vaginal application of IQB3002, a dual-chamber microbicide gel containing the nonnucleoside reverse transcriptase inhibitor IQP-0528 in rhesus macaques. Antimicrl Agents Chemothe. 2016;60(3):1393-400.
- Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir based preexposure prophylaxis for HIV infection among African women. New England J Medici. 2015;372(6):509-18.
- Vysloužil J, Kubová K, Vetchý D et al. Clinical testing of antiretroviral drugs as a future prevention against vaginal and rectal transmission of HIV infection–review of currently available results. Acta Pharmace. 2019;69(3):297-319.
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 3;329(5996):1168-74.
- Van der Straten A, Stadler J, Montgomery E, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PloS one. 2014;9(2):e89118.
- Zhang W, Hu M, Shi Y, et al. Vaginal microbicide film combinations of two reverse transcriptase inhibitors, EFdA and CSIC, for the prevention of HIV-1 sexual transmission. Pharmace Res. 2015;32:2960-72.
- Grammen C, Van den Mooter G, Appeltans B, et al. Development and characterization of a solid dispersion film for the vaginal application of the anti-HIV microbicide UAMC01398. Inter J Pharmace. 2014;475(1-2):238-44.
- Peitzmeier SM, Tomko C, Wingo E, et al. Acceptability of microbicidal vaginal rings and oral pre-exposure prophylaxis for HIV prevention among female sex workers in a high-prevalence US city. AIDS care. 2017;29(11):1453-7.
- Clark JT, Clark MR, Shelke NB, et al. Engineering a segmented dual-reservoir polyurethane intravaginal ring for simultaneous prevention of HIV transmission and unwanted pregnancy. PloS one. 2014;9(3):e88509.
- Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. England J Medic. 2016;375(22):2121-32.
- Buckheit Jr RW, Watson KM, Morrow KM, et al. Development of topical microbicides to prevent the sexual transmission of HIV. Antiv Res. 2010;85(1):142-58.
- Mankar SD, Rach PR. Solubility enhancement of poor water soluble drugs by solid dispersion: A review. J Drug Deliv Therape. 2018;8(5):44-9.
- Khuspe PR, Kokate K, Mandhare T, et al. The comprehensive review on solid dispersion technology.
- Breitenbach J. Melt extrusion: from process to drug delivery technology. Euro J Pharmac Biopharmace. 2002;54(2):107-17.
- Chokshi R, Zia H. Hot-melt extrusion technique: A review. Iran J Pharmace Res. 2004;3(1):3-16.
- Perissutti B, Newton J.M., Podezeck F., et al. Preparation of extruded Carbamazepine and PEG 4000 as a potential rapidrelease dosage form. Europ. J. Pharmaceut Biopharmaceut., 53: 125-132.
- Vilhelmsen T, Eliasen H, Schæfer T. Effect of a melt agglomeration process on agglomerates containing solid dispersions. Inter J Pharmac. 2005;303(1-2):132-42.
- Tsinontides SC, Rajniak P, Pham D, et al. Freeze drying principles and practice for successful scale-up to manufacturing. Inter J Pharma. 2004;280(1-2):1-6.
- Seo A, Schafer T. Melt agglomeration with polyethylene glycol beads at a low impeller speed in a high shear mixer. Euro J Pharmac Biopharmac. 2001;52(3):315-25.
- Allen Jr LV. Pharmaceutical dosage forms and drug delivery systems.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref