Research Article - Journal of Veterinary Medicine and Allied Science (2018) Volume 2, Issue 1
Preliminary survey on influence of renal portal system during propofol anesthesia in yellow-bellied turtle (Trachemys scripta scripta).
Marco di Giuseppe1*, Marco Luparello1, Bernadette Nastasi2, Filippo Spadola21Centro Veterinario per Animali Esotici, Palermo, Italy
2Department of Veterinary Science, Veterinary Teaching Hospital, University of Messina, Messina, Italy
- *Corresponding Author:
- Giuseppe MD
Veterinary Center for Exotic animals
Palermo
Italy
Tel: + 06-3333208
E-mail: marcodigiuseppe@yahoo.com
Accepted Date: April 07, 2018
Citation: Giuseppe MD, Luparello M, Nastasi B, et al. Preliminary survey on influence of renal portal system during propofol anesthesia in yellow-bellied turtle (Trachemys scripta scripta). J Vet Med Allied Sci 2018;2(1):12-5.
Abstract
Renal portal system makes challenging administration of drugs in reptiles. In fact renal portal system can accelerate excrection, increase clearance and consequently decrease plasmatic drug levels of drugs administered in the caudal quarter of the body. Fourteen healthy adult yellow-bellied sliders were used for this study. Turtles were randomly divided into three groups (A,B,C). A dose of 5 mg/kg of propofol was administered through occipital venous sinus (A), through subcarapacial venous sinus (B), and through coccygeal vein (C). Induction time, tracheal tube insertion time, surgical plane of anesthesia and full recovery time were recorded. Heart rate and respiratory rate were recorded for thirty minutes. The quality of anaesthesia was recorded as sedation score (ranging from 0 to 4). While in group A surgical level of Anastasia was reached, in the group B anesthesia induction was never achieved and only in two turtles of group C, surgical plane of anesthesia was achieved just in the hindquarter; these turtles showed full motility in forelimbs and head. No significant differences were statistically noted in heart rate when mean values were compared between groups. Time of full movement recovery of group C were significantly high, if compared with values of group A. Possible causes of failure to reach anaesthesia level in group B may be caused to a dosetoo low for this route of administration. Failure to obtain general anaesthesia in group C may suggest an influence of the renal portal system, but it does not explain limited effect just on hind limbs. In some subjects of the group C, elimination of the drug was very long, but recovery was uneventful. Propofol in yellow-bellied turtles showed altered mechanisms of action depending on the injection sites.
Keywords
Propofol, Turtles, Renal Portal system, Anaesthesia, Intravenous.
Introduction
Chelonians have a renal portal system that consists in a ring of vessels around kidney, including cranial portal vein and caudal portal vein. Blood flows from the caudal area through coccygeal and iliac veins, than it continues into afferent renal portal vein, which transfers blood to the kidneys [1,2]. Blood perfusing tubules leaves kidney by efferent portal vein, which join post-cava vein. Portal renal system is aimed to perfuse kidney during dehydration period, ensuring adequate perfusion into renal tubules when glomerular flow is reduced, and consequently avoiding ischemic damages. [3,4]. Depending on hydration status, blood from caudal portion can flow directly to kidneys or may bypass it and flow directly to the circulatory system. This apparatus is ruled by a system of valves located between the abdominal vein and the femoral vein. Renal portal system makes challenging administration of drugs in reptiles.
Common advices is to administered drugs at the cranial quarter of the body, in order to avoid renal portal system that may accelerate excretion, increase clearance and consequently decrease plasmatic drug levels. From the other side several studies showed that clinical relevance of this concern is minimal and that probably renal portal system may have greater interaction merely in dehydrated animals [5,6].
Contrariwise, a research on administration in cranial or caudal quarter of the same dosage of dexmedetomidine and ketamine mixture, showed a strong difference in sedation power [7]. Clinical evidence is missing, therefore drugs administration is still advised on cranial quarter [8]. The aim of this study is to evaluate the potential influence of renal portal system during propofol anaesthesia in freshwater turtles.
Material and Methods
Fourteen healthy adult yellow-bellied sliders (Trachemys scripta scripta; mean weight of 898 ± 442.13 g were used for this study. Turtles were anesthetized to perform a complete clinical examinations and safely collect a pharyngeal swabs. Most of these turtles were private owned animals, referred to an exotic and wild animal practice.
Owners signed an informed consent, and the study was performed in compliance with directive 2010/63/EU. Clinically unhealthy turtles were excluded from this study. Turtles were acclimatized one day before procedures in an environmental-controlled tank (25°C of water temperature and 27°C air temperature).
Turtles were randomized divided into three groups. In the group A, drug was administered through occipital venous sinus. In the group B, drug was administered through caudal subcarapacial plexus. In the group C, drug was administered through coccygeal vein.
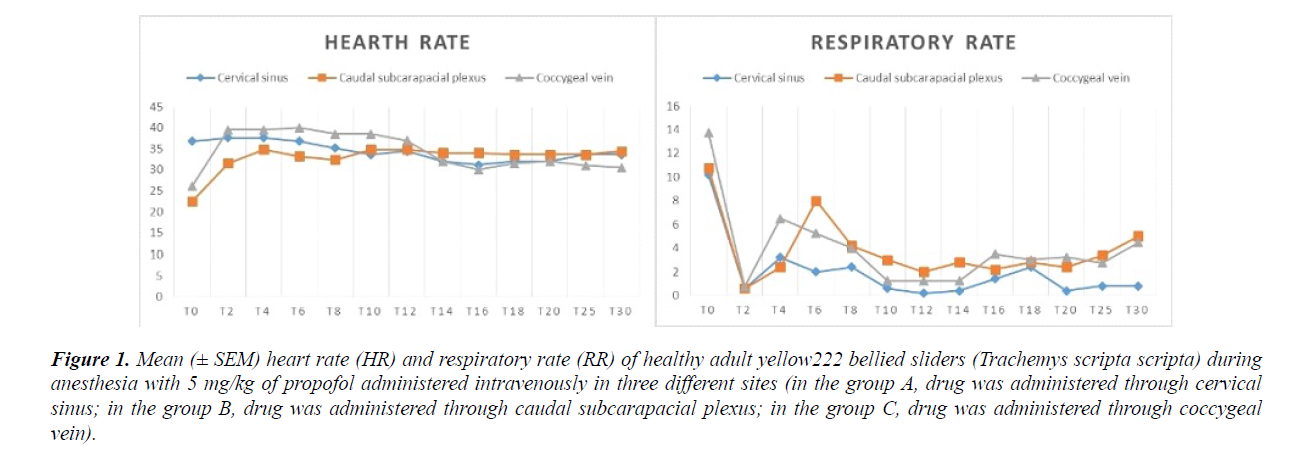
A dose of 5 mg/kg of propofol (Vetofol 1.0%, Norbrook, UK) was used for each turtle. Slow movements, pinching reflex lost, mandibular tone lost, mandibular tone recovery, pinching reflex recovery and full movements recovery were recorded. The time from the drug administration to the loose of pinching reflex was recorded as induction time. The time from the drug administration to the loose of the mandibular tone was recorded as tracheal tube insertion time. The time from induction until the return of the pinching reflex, was recorded as surgical plane of anesthesia. The time from the induction until full movements returned was recorded as full recovery time. Heart rate and RR were assessed at T (2), T (4), T (6), T (8), T (10), T (12), T (14), T (16), T (18), T (20), T (25) T (30), although full recovery occurred in shorter or longer time. Quality of anesthesia (SS) was recorded as the anesthesia score from 0 to 4 (Table 1).
Table 1. Quality of anesthesia was evaluated through a sedation scores.
| 0 | For fully active animal, without anesthesia |
| 1 | Head and neck withdrawal reflex being delayed |
| 2 | Toe pinch reflex being delayed but palpebral reflex still present |
| 3 | Disappearance of the palpebral reflex and biting reflex being delayed |
| 4 | Disappearance of the biting reflex, tracheal tube insertion possible |
The study was performed in compliance 167 with directive 2010/63/EU of the European parliament and of the Council of the European Union.
Descriptive statistical analysis and two-ways ANOVA test followed by Bonferroni test, were performed using Graphpad Prism 4.03 (GraphPad Software, Inc., USA). A value of p<0.05 was considered to be significant.
Results
Mean millilitres of injected anaesthetic was of 0.445 ± 0.21 mL. Basal hearth rates were 36.8 ± 4.60 bpm in the group A, 22.4 ± 9.63 bpm in the group B, and 26 ± 12 bpm in the group C. Basal respiratory rates were 10.2 ± 2.92 58 breaths per minutes (brpm) in the group A, 10.8 ± 2.7.
brpm in the group B, and 13.75 ± 1.70 brpm in the group C. Anaesthetic results are reported in Table 2.
Table 2. Recoded anaesthetic parameters in all gropus (A, B, C).
| Recorded parameters | A group | B group | C group |
|---|---|---|---|
| Slow movements appearance | 1.16 ± 0.56 minutes | Never | 3.75 ± 3.59 minutes |
| Induction time | 1.8 ± 30.8 minutes | Never | 4.20 ± 3.39 minutes |
| Tracheal tube insertion time | 3.89 ± 2.45 minutes. | Never | Never |
| Surgical plane of anesthesia | 14.66 ± 2.14 minutes | Never | 10.27 ± 4.62 minutes |
| Full recovery time | 26 ± 9.87 minutes | Never | 653 ± 429.92 minutes |
| Max sedation score | 3 | 0 | 1 |
In the group A, slow movements occurred on average of 1.16 ± 0.56 minutes. Mean induction time was within 1.8 ± 30.8 minutes. Mean tracheal tube insertion time was within 3.89 ± 2.45 minutes. In one female terrapin (1660 g) the mandibular tone was not lost. Time of surgical plane of anesthesia was on average of 14.66 ± 2.14 minutes, and full recovery time occurs on average of 26 ± 9.87 minutes. Mean sedation score achieved was 2.4 ± 0.54 (max value was 3). In the group A, turtles were intubated and ETCO2 was recorded, within an average of 8.96 ± 12.56 mmHg during whole procedures.
In the group B anesthesia induction was never achieved (SS=0).
In the group C mandibular tone was never lost in all anesthetized turtles. Moreover, in two specimens (2 female, 1.174 g; 742 g), surgical plane of anesthesia were achieved just in the hindquarter; these turtles shown a full motility in the forelimbs and head. Slow movements occur on average of 3.75 ± 3.59 minutes. Mean induction time was within 4.20 ± 3.39 minutes.
Time of surgical plane of anesthesia was on average of 10.27 ± 4.62 minutes. Full recovery time occurs on average of 653 ± 429.92 minutes. Mean sedation score achieved was 0.75 ± 0.5 (max value was 1). All recorded HR and RR values are reported in Figure 1.
Intra group statistics did not shown significant difference in HR values, while in group A, a statistical difference was noted when RR values at T (0) were compared with all other values, except to values recorded at T (4). In group B, a statistical difference was noted when RR values at T (0) were compared with all other values, except to values recorded at T (6). In group C, a statistical difference was noted when RR values at T (0) were compared with all other values, except to values recorded at T (4). No significant differences were statistically noted in HR when mean values were compared between groups. Respiratory rate values were significantly high (p<0.05) at T (6), when values of group A were compared with values of group B. The time of full movement recovery of group C were significantly higher (p<0.05), if compared with values of group A. All others values did not showed any statistical significant difference.
Discussion
Propofol is largely used in reptile medicine. Countless studies reported a well-knowed and appreciated an aesthetic efficacy, a rapid induction time and a short recovery period [9-15].
A current study advised the possibility of intracoelomic administration in red eared slider [16] but recommended administration sites are the jugular vein, and the brachial plexus. Recently a bilateral hind limbs paresis after an accidental sub meningeal injection of propofol into the subcarapacial venous sinus was reported [17] and this administration site is widely avoided even if evidence is missing. In our study all selected administration sites were easily accessible.
Obtained results with this study did not outperform by aforementioned studies, but some recorded data were unexpected.
In the group A full an aesthetic plan was easily reached in all turtles. Contrariwise, in other groups no signs of deep sedation were achieved. Different hypotheses can be issued.
Possible causes of failure to obtain an anesthetic plan after propofol administration into caudal subcarapacial plexus (group B) are not clear. Propofol in all probability bypassed the systemic circulation through renal portal system and it was promptly eliminated. A higher dose is probably required for this administration route. In a study conducted on eared slider an adequate sedation was indeed obtained by using doses ranging between 10 and 20 mg/kg of propofol [18] while in our study a dose of 5 mg /kg was used.
A statistically significant increase in respiratory rate was noted at six minutes after drug administration in animals of group B. We can assume that respiratory rate increasing is related to the various handling phases performed in order to assess heart rate, consequently that there is no connection with drug effect.
Even though not statistically significant, respiratory and heart rates suddenly changed at T2 (Figure 1).
Pain caused by injection procedure, can be deducted by increased heart and respiratory rate. Obviously, since physiological characteristics of treated species, we cannot admit that successively apnoea was due to direct action of drug.
Drug administration at the level of coccygeal vein (group C), did not give a satisfactory status of anesthesia; in 50% of treated turtles was obtained a phase of surgical anesthesia only in the hindquarters (hind legs, tail). Failure to general sedation may perhaps suggest an influence of the renal portal system. However it does not explain limited effect on hind limbs, because propofol would have acted producing a little systemic effect. A further hypothesis is that propofol was injected into the intrathecal space [19]. However, our results are in contrast with a previous report, describing complete paralysis of fore and hind limbs, coma and spinal necrosis [17]. This did not happened in treated turtles, in fact elimination of drug was very long in some animals (approximately 9 hours), but recovery was uneventful. Ultimately we may assume that propofol in reptiles have altered mechanisms of action and among these even a local action. Our assumptions need to be further investigated by a systematic study of on pharmacodynamics of propofol in different injection sites on freshwater turtles.
References
- Murray MM. Cardiopulmunary Anatomy and Physiology in Mader D. Reptile Medicine and Surgery. 2006;124-8.
- O'Malley B. Tortoises and Turtles, in O'Malley B. Clinical Anatomy and Physiology of Exotic Species. 2005;41-56.
- Holz P, Barker IK, Crawshaw GJ, et al. The Anatomy and Perfusion of the Renal Portal System in the Red-Eared Slider (Trachemys scripta elegans). J Zoo Wildl Med. 1997;28:378-85.
- Holz P, Raidal S. Comparative Renal Anatomy of Exotic Species. Vet Clin North Am Exot Anim Pract. 2006;9:1-11.
- Holz P, Barker IK, Burger JP, et al. The Effect of the Renal Portal System on Pharmacokinetic Parameters in the Red-Eared Slider (Trachemys scripta elegans). J Zoo Wildl Med. 1997;28:386-93.
- Giorgi M, Salvadori M, De Vito V, et al. Pharmacokinetic/Pharmacodynamic Assessments of 10 Mg/Kg Tramadol Intramuscular Injection in Yellow-Bellied Slider Turtles (Trachemys Scripta Scripta). J Vet Pharmacol Ther. 2015;38:488-96.
- Lahner L, Mans C, Sladky KK. Comparison of Anesthetic Induction and Recovery Times after Intramuscular, Subcutaneous or Intranasal Dexmedetomidine-Ketamine Administration in Red-Eared Slider Turtles (Trachemys Scripta Elegans). 2011;136-7.
- Sladky KK, Mans C. Clinical Anesthesia in Reptiles. J Exot Pet Med. 2012;21:17-31
- Bennet RA, Schumacher J, Hedjazi Haring K, et al. Cardiopulmunary and Anesthetic Effects of Propofol Administreted in Intraosseously to Green Iguana. J Am Vet Med Ass. 1998;212:93-8.
- Schumacher J, Yelen T. Anesthesia and Analgesia, in Mader D. Reptile Medicine and Surgery. 2006;442-52.
- MacLean RA, Harms CA, Braun-McNeill J. Propofol Anesthesia in Loggerhead (Caretta Caretta) Sea Turtles. J Wildl Dis. 2008;44:143-50.
- Santos AL, Bosso AC, Alves Júnior JR, et al. Pharmacological restraint of captivity giant amazonian turtle Podocnemis expansa (testudines, podocnemididae) with Xylazine and Propofol. Acta Cir Bras. 2008;23:270-3.
- McFadden MS, Bennett RA, Reavill DR, et al. Clinical and Histologic Effects of Intracardiac Administration of Propofol for Induction of Anesthesia in Ball Pythons (Python Regius). J Am Vet Med Assoc. 2011;239:803-7.
- Alves-Júnior JR, Bosso AC, Andrade MB, et al. Association of Acepromazine with Propofol in Giant Amazon Turtles Podocnemis Expansa Reared in Captivity. Acta Cir Bras. 2012;27:552-6.
- Bertelsen MF, Buchanan R, Jensen HM, et al. Assessing the Influence of Mechanical Ventilation on Blood gases and Blood pressure in rattlesnakes. Vet Anaesth Analg. 2015;42:386-93.
- Schroeder CA, Johnson RA. The Efficacy of Intracoelomic Fospropofol in Red-Eared Sliders (Trachemys Scripta Elegans). J Zoo Wildl Med. 2013;44:941-50.
- Quesada RJ, Aitken-Palmer C, Conley K, et al. Accidental Submeningeal Injection of Propofol in Gopher Tortoises (gopherus polyphemus). Vet Rec. 2010;167:494-5.
- Ziolo MS, Bertelsen F. Effects of Propofol administered via the Supravertebral sinus in Red-Eared Sliders. J Am Vet Med Assoc. 2009;234:390-93.
- Mans C. Clinical Technique: Intratechal Drug administration in Turtles and Tortoises. J Exot Pet Med. 2014;23:67-70.