Research Article - Biomedical Research (2017) Volume 28, Issue 10
Preliminary evaluation of proprotein convertase subtilisin/kexin type 5 mutations in lower extremity artery disease
Setalia Popa, Viviana Aursulesei*, Eusebiu Vlad Gorduza and Irina Iuliana CostacheGrigore T. Popa University of Medicine and Pharmacy, Sf. Spiridon Emergency Districtual Clinical Hospital, Romania
- *Corresponding Author:
- Viviana Aursulesei
Grigore T. Popa University of Medicine and Pharmacy
Sf. Spiridon Emergency Districtual Clinical Hospital, Romania
Accepted on March 8, 2017
Abstract
The low level of high-density lipoprotein cholesterol (HDL-c) is a major risk factor for atherosclerotic cardiovascular disease including Lower extremity artery disease (LEAD). The variability of proprotein convertase subtilisin/kexin type 5 (PCSK5) gene was proposed as regulator of HDL-c metabolism. The molecular mechanisms are not fully elucidated. Several studies suggest that several genetic anomalies could influence the risk of developing LEAD. Considering these data, the aim of our study was to identify PCSK5 gene variants in patients with LEAD using multiplex ligation-dependent probe amplification (MLPA) as a screening method. The study included 48 patients with LEAD documented by angiography. All patients had low levels of HDL-c and a high total cholesterol/HDL-c ratio. DNA extraction was performed using the precipitation method, while polymorphisms of all PCSK5 gene exons were assessed using MLPA. The MPLA results were subsequently analysed with Coffalyser® software. Analysis of PCSK5 gene expression showed normal genotype in 45 patients. Heterozygous deletion of exon 9 of PCSK5 gene was identified in only 3 patients and was not correlated with HDL-c values. Our data cannot establish a clear correlation between mutational profile of PCSK5 gene and LEAD, so more extensive genetic testing in larger cohorts are required.
Keywords
Proprotein convertase subtilisin/kexin type 5 gene, Lower extremity artery disease, HDL-cholesterol, Atherosclerosis.
Introduction
Atherosclerosis is a progressive inflammatory disease characterized by the accumulation of lipoproteins, mononuclear cells, smooth muscle cells and extracellular matrix in the arterial wall [1]. It is known that low levels of high-density lipoprotein cholesterol (HDL-c) represent a major risk factor for atherosclerotic cardiovascular disease including lower extremity artery disease (LEAD) [2]. Several experimental and human studies suggested the involvement of genetic alterations of proprotein convertase subtilisin/kexins (PCSKs), a serine endopeptidase family, in the metabolism of HDL-c, very low-density lipoprotein and chylomicrons [3-6]. The members of endopeptidase family are precursors of biologically active products and often interact with rennin through a mechanism not fully elucidated [7]. PCSK type 5 (PCSK5), as well as PCSK type 3 and PCSK type 6, acts on endothelial and lipoprotein lipases that modulates HDL-c levels [8]. PCSK5 is a precursor protein with 1860 amino acids (206.942 Da) and two isoforms, and is encoded by PCSK5 gene located on chromosome 9 (78505560-78977255 bp from p-terminal end (9q21.13-31)) [9]. In patients with impaired HDL-c levels seven variants of PCSK5 gene were detected, but none of these variants was a missense mutation [10]. Some experimental and clinical studies have identified PCSK5 in the atherosclerotic plaque and suggested that PCSK5 modulates the inflammation in atherosclerosis. It has been shown that PCSK5 activates matrix metalloproteinases and integrins, while the levels of PCSK expression increase during macrophage differentiation [11]. In contrast, Tampere Vascular Study, a unique study by its design, based on collection and comparative analysis of human atherosclerotic plaques in different vascular territories and control arteries, suggested that PCSK5 is significantly downregulated [12]. Similarly, Turpeinen et al. have shown that the PCSK5 gene is under expressed in smooth muscle cells of atherosclerotic plaques, but this seems to be not specific for atherosclerotic lesions [13]. On the other hand, recent sophisticated genetic approaches consider that the variation in the genetic code and its interaction with environmental factors may also be involved in the pathogenesis of LEAD [14]. Taking into account the above considerations, the connection between PCSK5 expression and atherosclerotic vascular disease such as LEAD is not fully elucidated and should be clarified in further studies. This study aimed to identify PCSK5 gene variants in patients with LEAD using multiplex ligation-dependent probe amplification (MLPA) as a screening method.
Materials and Methods
Our study included 48 patients (41 men and 7 women, aged between 45-85 years) admitted to the Cardiology Department, “Sf. Spiridon” Emergency Districtual Clinical Hospital Iasi between July and December 2012. All patients came from the North-East region of Romania. The study was performed in accordance with the ethical standards of the institutional committee. The patients were previously informed about the purpose of the study and were included after signing an informed consent. The patients eligible for the study had LEAD documented by angiography. Dyslipidemia was confirmed by a fasting lipid profile analysis (cholesterol, HDLcholesterol, LDL-cholesterol and triglycerides).
A blood sample was also taken for genomic Deoxyribonucleic Acid (DNA) extraction. The exons of PCSK5 gene were analysed in DNA. The extractions were carried out by using Wizard® Genomic DNA Purification-Promega Kit (Promega Inc., Madison, WI, USA). The DNA was quantified using a Nanodrop 2000® spectrophotometer and then aliquoted and labeled in order to achieve DNA collection in Medical Genetics Department.
The gene sequences were amplified using MLPA technique for PCSK5 exons, with a 60 ng/μl DNA concentration. The technique was performed using P273-A1 PCSK5 MRC kit (provided by MRC-Holland). It contains 48 samples consisting of amplicones between 130 and 493 nucleotides and 9 control sequences with amplification products of 120 nucleotides (4 sequences check DNA quality, 3 sequences for DNA control and 2 sequences identify the gender of patient). The MLPA reaction was divided into five steps: DNA denaturation, hybridization and ligation, PCR amplification, capillary electrophoresis and data analysis. The Cy5 fluorochrome with absorption at 649 nm and emission at 670 nm was used. The fluorescence level was measured by spectrophotometry and the results were further analysed using Coffalyser® software.
Results
Most of our patients were males (85% of cases) and smokers (81% of patients) with a mean age of 66.5 ± 1.45 years and no significant demographic differences between rural and urban residences. The lipid profile analysis detected the following mean values: total cholesterol of 213.7 ± 7.2 mg/dL, LDLcholesterol of 137.2 ± 9.5 mg/dL, HDL-cholesterol of 38.2 ± 5.1 mg/dL, triglycerides of 165.1 ± 11.4 mg/dL.
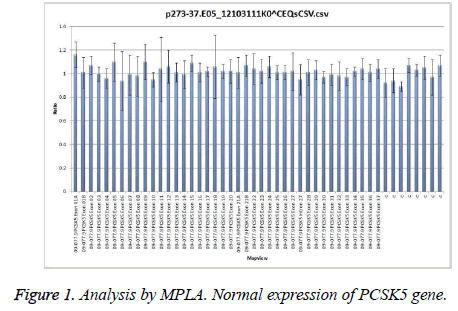
MPLA gene analysis showed a normal amplification in 45 patients (Figure 1), with values ranging between 0.8 and 1.2. Thus, we considered that all these patients were homozygotes for normal alleles of PCSK5 gene.
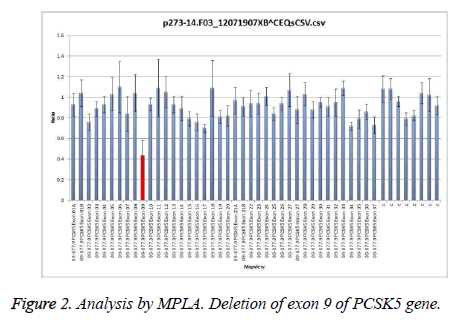
Three of our patients (6.25%) had heterozygous deletion of exon 9 of PCSK5 gene as shown in Figure 2.
The three patients belonged to different families; they were aged over 60 years, and had low HDL-c levels. One of the patients was female and non-smoker. It appeared that these patients were carriers of the same mutation. We divided the patients according to the presence/absence of the described mutation. In order to assess the role of the proprotein convertase in regulating HDL-c levels in the two groups, we applied the Student’s t-test. No significant differences between the two groups were found (p=0.052), so the null hypothesis is true.
Discussion
Lower extremity artery disease is a significant public health problem that impairs quality of life, as well as a major cause of cardiovascular morbidity and mortality [15]. Genetic investigation of LEAD is an evolving concept useful for understanding its pathogenesis and for identification of new therapeutic targets. Several studies suggest that the risk of developing LEAD is influenced by additional factors such as genetic anomalies and unstudied environmental components [14]. The study of PCSK5 gene is justified by its role in HDL-c metabolism, a major risk factor for LEAD. Thus, the detection of gene mutations could be correlated with the risk of developing or progression of the disease. It was suggested that some polymorphisms of PCSK5 gene could be associated with a variable expression that generates mild forms of atherosclerotic disease [16]. The expression of PCSK5 gene in atherosclerotic plaque is also important, because endothelial lipase has the maximum concentration in vascular endothelium, and its inhibition by the proprotein convertase would acts directly on the endothelial lipase. For example, Marchesi investigated the specific function of PCSK5 in endothelial cells and showed that knockout rats for PCSK5 gene mutations appeared more likely to develop the vascular ageing lesions than did the normal rats [17]. Cardiovascular ageing is known to be significantly associated with arterial stiffness and a reduced arterial diameter, caused by interstitial collagen deposition, leading to vascular disease [17]. Our study aimed to analyse PCSK5 gene mutations in patients with documented atherosclerotic LEAD by using a genetic screening method that identifies the number of copies of gene sequences. MLPA technique identified a heterozygous deletion of exon 9, namely loss of one half of amplified gene alleles. This lack of amplification may be caused by several conditions: complete exon deletion, partial exon deletion, exon mutations (point mutations, deletions, insertions, duplications, translocations, etc.) [18,19]. The mutation identified in exon 9 of PCSK5 gene was not associated with HDL-c levels. Although in our study the carriers of mutation were not related, they might have relatives with the same anomaly. This finding may be a starting point to establish if this mutation could be a family characteristic (through descendants). The study included only patients from the North-East region of Romania. Thus, it is interesting to find out if the discovered mutation could be a genetic feature for LEAD in this population. Secondly, the discovered gene mutation is present in subjects with advanced LEAD. It would be interesting to expand the study in patients with asymptomatic disease or early clinical manifestations. Thirdly, it is important to detail other aspects related to PCSK5 mutation such as cleavage of protein, interactions with the substrate, as well as the variability of expression levels in pathological conditions (e.g. interaction with growth factors receptors and enzymes). Also, MLPA is a fast method of screening but it requires confirmation by other methods such as PCR, qPCR, FISH, Southern blot etc. By using a confirmation method it is possible to exclude the presence of one of the three normal variants (polymorphisms) of PCSK5 gene described in literature [6,16,17]. Previous studies have documented the polymorphisms of exons 1, 12, 18, and introns 4, 7, 8, 11, 16, 17, 19, 20 in human atherosclerotic lesions [16]. Thus, the presence of this mutation should be addressed in studies by using other molecular techniques for an optimal characterization. In conclusion, the clinical significance of our data requires further studies using more complex genetic testing in larger cohorts.
Acknowledgements
This study is part of PhD thesis “Modern therapeutic approaches in lower extremity artery disease” and it was not supported financially.
References
- Leschka S, Seitun S, Dettmer M, Baumuller S, Stolzmann P, Goetti R, Scheffel H, Feuchtner G, Wunnicke K, Wildermuth S, Oehlschlegel C, Marincek B, Jochum W, Alkadhi H. Ex vivo evaluation of coronary atherosclerotic plaques: characterization with dual-source CT in comparison with histopathology. J Cardiovasc Comput Tomogr 2010; 4: 301-308.
- Alagona C, Soro A, Westerbacka J, Ylitalo K, Salonen JT, Salonen R, Yki-Järvinen H, Taskinen MR. Low HDL cholesterol concentration is associated with increased intima-media thickness independent of arterial stiffness in healthy subjects from families with low HDL cholesterol. Eur J Clin Invest 2003; 33: 457-463.
- Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov 2012; 11: 367-383.
- Boes E, Coassin S, Kollerits B, Heid IM, Kronenberg F. Genetic epidemiological evidence on genes associated with HDL cholesterol levels: a systematic in-depth review. Exp Gerontol 2009; 44: 136-160.
- Dannunzio V, Donato M, Buchholz B, Perez V, Miksztowicz V. High cholesterol diet effects on ischemia-reperfusion injury of the heart. Can J Physiol Pharmacol 2012; 90: 1185-1196.
- Breslow JL. Genetics of lipoprotein abnormalities associated with coronary artery disease susceptibility. Ann Rev Genet 2000; 34: 233-254.
- Liu J, Afroza H, Rader DJ, Jin W. Angiopoietin-like protein 3 inhibits lipoprotein lipase activity through enhancing its cleavage by proprotein convertases. J Biol Chem 2010; 285: 27561-27570.
- Choi S, Korstanje R. Proprotein convertases in high-density lipoprotein metabolism. Biomark Res 2013; 1: 27.
- van de Loo JW, Creemers JW, Kas K, Roebroek AJ, Van de Ven WJ. Assignment of the human proprotein convertase gene PCSK5 to chromosome 9q21.3. Cytogenet Cell Genet 1996; 75: 227-229.
- Iatan I, Dastani Z, Do R, Weissglas-Volkov D, Ruel I, Lee JC, Huertas-Vazquez A, Taskinen MR, Prat A, Seidah NG, Pajukanta P, Engert JC, Genest J. Genetic variation at the proprotein convertase subtilisin/kexin type 5 gene modulates high-density lipoprotein cholesterol levels. Circ Cardiovasc Genet 2009; 2: 467-475.
- Stawowy P, Fleck E. Proprotein convertases furin and PC5: targeting atherosclerosis and restenosis at multiple levels. J Mol Med 2005; 83: 865-875.
- Oksala N, Levula M, Pelto-Huikko M, Ortiz RM, Järvinen O, Salenius JP, Ozsait B, Komurcu-Bayrak E, Erginel-Unaltuna N, Huovila AP, Kytomaki L, Soini JT, Kahonen M, Karhunen PJ, Laaksonen R, Lehtimäki T. Carbonic anhydrases II and XII are up-regulated in osteoclast-like cells in advanced human atherosclerotic plaques-Tampere Vascular Study. Ann Med 2010; 42: 360-367.
- Turpeinen H, Raitoharju E, Oksanen A, Oksala N, Levula M. Proprotein convertases in human atherosclerotic plaques: the overexpression of FURIN and its substrate cytokines BAFF and APRIL. Atherosclerosis 2011; 219: 799-806.
- Leeper NJ, Kullo IJ, Cooke JP. Genetics of peripheral artery disease. Circulation 2012; 125: 3220-3228.
- Eraso LH, Fukaya E, Mohler ER, Xie D, Sha D, Berger JS. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol 2014; 21: 704-711.
- Cao H, Mok A, Miskie B, Hegele RA. Single-nucleotide polymorphisms of the proprotein convertase subtilisin/ kexin type 5 (PCSK5) gene. J Hum Genet 2001; 46: 730-732.
- Marchesi C, Essalmani R, Lemarie CA, Leibovitz E, Ebrahimian T, Paradis P, Seidah NG, Schiffrin EL, Prat A. Inactivation of endothelial proprotein convertase 5/6 decreases collagen deposition in the cardiovascular system: role of fibroblast autophagy. J Mol Med 2011; 89: 1103-1111.
- Gaudet P, Livstone MS, Lewis SE, Thomas PD. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief Bioinform 2011; 12: 449-462.
- van Eijk R, Eilers PH, Natte R, Cleton-Jansen AM, Morreau H, van Wezel T, Oosting J. MLPAinter for MLPA interpretation: an integrated approach for the analysis, visualisation and data management of Multiplex Ligation-dependent Probe Amplification. BMC Bioinformatics 2010; 11: 67.