Research Article - Biomedical Research (2017) Volume 28, Issue 12
Posterolateral discectomy and interbody fusion in the treatment of thoracic disc herniation
Jian Zhang#, Wei-Dong Liang#, Wei-Bin Sheng*, Hai-Long Guo and Qiang DengDepartment of Spine Surgery, the First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Wei-Bin Sheng
Department of Spine Surgery
The First Teaching Hospital of Xinjiang Medical University, PR China
Accepted date: April 24, 2017
Abstract
The aim of this study was to evaluate the surgical safety and efficacy of the Posterolateral Interlaminar Approach (PIA) for treating Thoracic Disc Herniation (TDH). A total of 24 TDH cases were treated via PIA between January 2006 and August 2014. Japanese Orthopedic Association (JOA) scoring, Otani grading, and the Bridwell criteria were used to evaluate treatment efficacy. Twenty-four patients were followed up for between 6 months and 3.5 y, and were reviewed in the clinic at 3, 6, and 12 months after surgery. The preoperative JOA score was 4.4 ± 2.4 points, while postoperatively that at 3 months was 8.7 ± 2.3 points, and at the last follow-up was 9.0 ± 2.3 points. The comparisons over time were statistically significant (P<0.05). According to Otani grading, the treatment results of 9 cases were excellent, 11 cases were good, 2 cases were acceptable, and 2 cases were poor, with the excellent and good rate at 83.3%. All bone grafts achieved phase I healing within 9 months after surgery, and there were no occurrences of loosening of internal fixation, fracture, or segmental collapse during follow-up. PIA could obtain satisfactory clinical results in treating TDH.
Keywords
Thoracic disc herniation, Posterolateral approach, Surgical treatment, Efficacy evaluation.
Introduction
Thoracic Disc Herniation (TDH) is relatively rare in clinical practice compared to cervical and lumbar disc herniation, accounting for only about 4% of all disc herniation cases [1]. Of all patients with TDH, 70% are asymptomatic, but once symptoms appear, these often severely affect the patient's function. TDH normally shows no specific clinical features, making the diagnosis more difficult, which can easily lead to misdiagnosis and missed-diagnoses, resulting in a negative impact on treatment efficacy. With the progression and increased applications of CT, MRI and other imaging technologies, early diagnosis of TDH has been made possible [2]. If clearly diagnosed, patients with symptomatic TDH should undergo early surgical treatment. Key first described TDH in 1838, and posterior laminectomy was the first effective treatment method developed. In 1952, Logue found that most TDH patients exhibited aggravated neurological symptoms after posterior laminectomy [3]. Studies showed that the operation of removing the ventral spinal herniated disc in posterior laminectomy might cause mechanical damage to the spinal cord; additionally, it might disturb spinal blood flow, leading to spinal cord ischemia [4]. Thereafter, surgeons kept exploring a series of modified surgical approaches to treat TDH [5]. Even with the modified surgical procedures, as many as 5.5% of patients still exhibited aggravated postoperative neurological symptoms [6-11]. From January 2006 to August 2014, our hospital performed posterolateral discectomy and interbody fusion to treat 24 TDH patients, the results of which showed good results after 6 months to 3.5 y of follow-up.
Materials and Methods
General information
Between January 2006 and August 2014, 24 TDH patients including 15 males and 9 females aged 37 to 64 y, with a mean age of 45.3 y, were admitted into the First Affiliated Hospital of Xinjiang Medical University. Two patients had acute onset TDH with a clear history of trauma, while the rest exhibited insidious onset. The disease durations ranged from 4 months to 2 y, with an average of 14 months. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Xinjiang Medical University. Written informed consent was obtained from all participants.
Clinical manifestations
Two patients’ conditions were caused by chest and back injuries, and rapidly aggravated by heavy manual labor, with disease duration of less than six months. The rest of the patients exhibited no obvious provoking factors, with slow onset and progressive aggravation. The primary clinical manifestations were 18 cases of lower extremity numbness and difficulty in walking to various degrees, 9 cases of chest and back soreness and pain, 7 cases of thoracic and abdominal zonesthesia, 4 cases of intermittent claudication, 4 cases of intercostal neuralgia, and 2 cases of complete paraplegia. On physical examination, 19 cases had reduced sensation below the involved plane; 16 cases had increased muscle tone, hyperreflexia in the knee and ankle, and a pathological positive Babinski reflex; 4 cases had muscle atrophy and decreased muscle strength; 2 cases had reduced reflexes in the knee and ankle; 6 cases had urinary dysfunction; and 10 cases had a reduced abdominal reflex.
Imaging features
All patients underwent X-ray and MRI. Apart from the 2 cases with traumatic soft injury, patients all underwent CT scanning to further clarify the extent of disc calcification or ligamentous ossification, as well as the degree of thoracic spinal stenosis. All patients exhibited single segment herniation, with 16 cases of central herniation, 8 cases of para-central herniation, 18 cases of hard herniation, and 6 cases of soft herniation. Two cases exhibited herniation at T7~8, 4 cases at T8~9, 4 cases at T9~10, 5 cases at T10~11, and 9 cases at T11~12, among whom 3 cases were accompanied by thoracic ossification of the ligamentum flavum, and 2 cases were accompanied by thoracic ossification of the posterior longitudinal ligament.
Surgical methods
All of the 24 patients underwent posterolateral discectomy and interbody fusion. The herniated segment was determined based on preoperative imaging, and positioned by intraoperative Carm fluoroscopy. General anesthesia was induced, and patients were then placed in the prone position. With the affected segment at the center, a posteromedial incision was performed, with an incision width of about 8~10 cm, to reveal the spinous process, lamina, and zygapophysial joints of the segments adjacent to the herniated segment. Screws were placed on the pedicle of the vertebral arch adjacent to the affected segment, and unilateral or bilateral resection was performed to remove the lower 2/3 of the upper lamina and the upper 1/3 of the lower lamina and partial spinous process, according to the extent and type of compression at the herniated segment: if the herniation was para-central or soft, unilateral surgery was performed; if the herniation was central or associated with intervertebral disc calcification, bilateral surgery was performed, together with partial or total removal of the articular process to expose the endorachis and nerve roots. The outer edge of the endorachis was determined, and the posterolateral edge of the affected intervertebral fibrous annulus was incised. Unilateral or bilateral sneak removal was then performed to clean the tissues of the affected intervertebral disc. If the lesion involved disc calcification, a micro-bone knife was used to drill it open from the epidural edge, followed by carefully expanding and removing the tissues of the affected intervertebral disc. During surgery, endorachis should not be directly pinched to remove ventrally compressed tissues to avoid damaging the spinal cord. After cleaning the tissues of the affected intervertebral disc, the nerve dissector was used to carefully separate the ventrally herniated disc tissue from the endorachis, which was then carefully removed from the posterolateral side, or pushed forwards into the intervertebral space and removed with nucleus pulposus forceps. If osteophytes existed behind the vertebral body, then after cleaning the vertebral space, the nucleus pulposus forceps, gun-shaped rongeur or micro bone knife was used to carefully remove the osteophytes. After decompression, the nerve dissector was used to confirm that no compression existed inside the ventral side of spinal cord, the intervertebrally implanted autologous bone, or the intervertebral fusion cage, and then the connecting rod was placed for fixation. After completing hemostasis, a normal drainage tube was placed at the incision, and the wound was sutured layer by layer. For this study, we performed 5 unilateral surgeries and 19 bilateral surgeries.
Post-treatment processing
In addition to conventional infection prevention and symptomatic treatments, 80 mg of methylprednisolone was intravenously infused for three days. If the incision drainage was less than 50 ml, the drainage tube was pulled out and the X-ray rechecked; if no severe osteoporosis or cerebrospinal fluid leakage existed, the patient could perform ambulation with the protection of a brace.
Evaluation criteria
Modified JOA scoring (excluding the upper limb scores, with a total score of 11 points) was used to assess nerve function and the improvement rate between before and after surgery [12,13], where the improvement rate=(postoperative score-preoperative score)/(11-preoperative score) × 100%. Treatment efficacy was assessed using the Otani grading method [14], which graded results as excellent: without postoperative symptom, activities were completely normal; good: with mild weakness, spasm or ankylosis, could participate in daily work; general: symptoms were improved, with residual reflex pain and moderate weakness causing difficulties in participating in daily work; and poor: without postoperative improvement. For bone healing, we used the Bridwell criteria to evaluate the fusion efficacy [15]: Level I: bone fusion and remodeling were complete, with central trabecular continuity; level II: bones were integrated, but bone remodeling was not complete, without a lucent zone; level III: bones were complete, with a lucent area in the upper or lower region; and level IV: bones were collapsed and absorbed.
Results
Clinical data
The operative time was 1.5~2.5 h, with an average of 2 h. Blood loss was 150~250 ml, with an average of 200 ml, and no intraoperative blood transfusion was performed. The 24 patients were all followed up for 0.5 to 3.5 y, with an average of 2.1 y.
Clinical efficacy
All patients were reviewed in the clinical service at 3, 6 and 12 months after surgery. The symptoms were improved to varying degrees, and no aggravation of neurological symptoms or spinal instability occurred during follow-up. Preoperative JOA score was 4.4 ± 2.4; this increased to 8.7 ± 2.3 points at 3 months after surgery and 9.0 ± 2.3 points at the last follow-up. The differences between the scores at all different postoperative time points and those before surgery were statistically significant (P<0.05). The improvement rate at the last follow-up was 74.6% ± 16.6%. According to the Otani grading, 9 cases were classed as excellent, 11 cases as good, 2 cases as acceptable and 2 cases as poor, with the excellent and good rate at 83.3%. Among the two cases of paraplegia, one patient did not significantly recover, while the other case had recovered from Frankel grade A to grade C at the 6 month follow-up.
Imaging evaluation
Three months after surgery, the grafts had achieved level II or III healing at follow up. At 6 months after surgery, 6 cases exhibited level I healing, and the rest had reached Level II healing; at 9 months after surgery, almost all cases achieved level I healing. No cases of loosening of internal fixation, fracture, or segmental collapse occurred during follow-up.
Surgical complications
All patients successfully completed the surgery without nerve injury occurring. One patient developed fat liquefaction at the incision, but the rest of the incisions all exhibited phase I healing. Three cases received an intraoperative spinal injury. In two cases the endorachis was repaired intraoperatively and covered with autologous fat tissues; no cerebrospinal fluid leakage occurred after surgery. One case failed the intraoperative repair, so postoperative lumbar subarachnoid catheterization plus cerebrospinal fluid splitting was performed while the incision drainage tube was occluded; the patient showed a successful repair two days after.
Discussion
Pathological features of TDH
Although the incidence of TDH is low, it can occur in various thoracic segments, and is more common in the lower thoracic discs. In this study, 18 cases occurred in T9~12 (75% of cases), which is similar to those rates reported in the literature [16]. According to the herniated sites, TDH can be divided into central, para-central, lateral, and intradural types. Central and paracentral types accounted for about 70% of cases in this study. The 24 cases in this study were all of central and paracentral types.
Diagnosis of TDH
Pain is the most common initial symptom of TDH. In this study, 9 cases manifested with chest and back pain, and 4 cases manifested with intercostal neuralgia, accounting for 54.2%. Numbness or paresthesia is another common symptom, and 19 cases in this study were associated with these symptoms. Clinical examination usually revealed central or mixed nerve damage signs such as sensory disturbances, muscle weakness and so on below the involved plane.
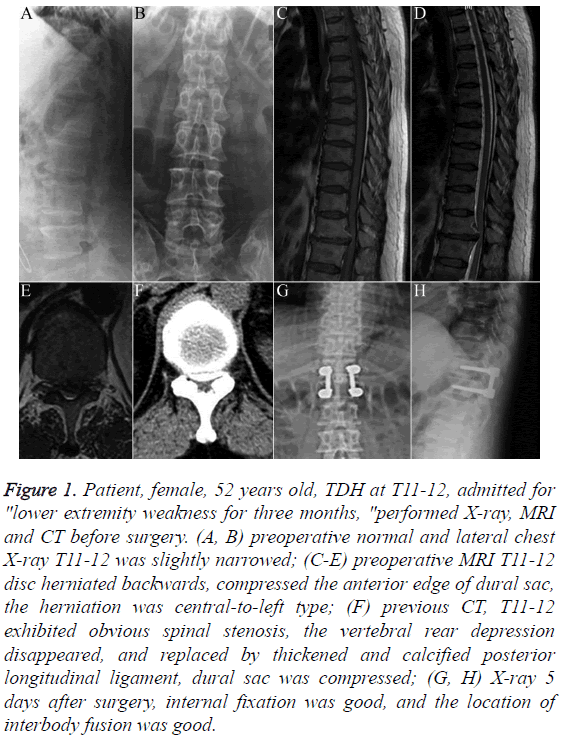
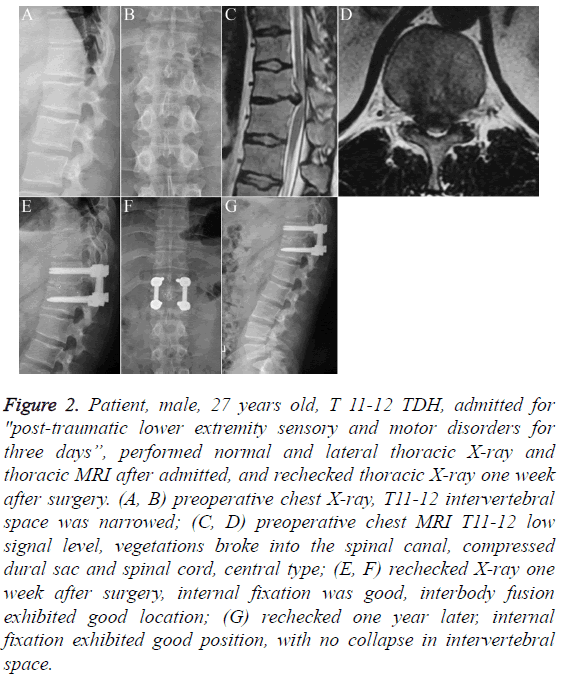
Imaging is an important diagnostic means. Plain X-ray has certain suggestive features towards the diagnosis of TDH, although this cannot be used to make the specific diagnosis. CT can display the sites and calcification conditions of TDH, and clearly show the proliferation of small joints, hypertrophy or ossification of the yellow ligament or posterior longitudinal ligament, and so on. Research has shown that in TDH, disc calcification could be as high as 30% to 70% [17], and this study had an intervertebral disc calcification rate of 75% (Figure 1). Traumatic TDHs mostly involve soft herniation, and the 2 cases of traumatic TDH in this study were both soft herniation (Figure 2). MRI is the most effective method to confirm the diagnosis of TDH [18]. It can directly display the compression of the spinal cord and spinal nerve by the affected disc, thus helping with classifying the lesion and selecting surgical methods. The 24 cases all underwent MRI, which clearly displayed the locations, extent and consolidated lesions of the TDH.
Figure 1: Patient, female, 52 years old, TDH at T11-12, admitted for "lower extremity weakness for three months, "performed X-ray, MRI and CT before surgery. (A, B) preoperative normal and lateral chest X-ray T11-12 was slightly narrowed; (C-E) preoperative MRI T11-12 disc herniated backwards, compressed the anterior edge of dural sac, the herniation was central-to-left type; (F) previous CT, T11-12 exhibited obvious spinal stenosis, the vertebral rear depression disappeared, and replaced by thickened and calcified posterior longitudinal ligament, dural sac was compressed; (G, H) X-ray 5 days after surgery, internal fixation was good, and the location of interbody fusion was good.
Figure 2: Patient, male, 27 years old, T 11-12 TDH, admitted for "post-traumatic lower extremity sensory and motor disorders for three days”, performed normal and lateral thoracic X-ray and thoracic MRI after admitted, and rechecked thoracic X-ray one week after surgery. (A, B) preoperative chest X-ray, T11-12 intervertebral space was narrowed; (C, D) preoperative chest MRI T11-12 low signal level, vegetations broke into the spinal canal, compressed dural sac and spinal cord, central type; (E, F) rechecked X-ray one week after surgery, internal fixation was good, interbody fusion exhibited good location; (G) rechecked one year later, internal fixation exhibited good position, with no collapse in intervertebral space.
Treatment of TDH
For the patients with no significant nerve damage, traditional treatment could be performed. The specific measures include rest, wearing braces, medication and functional exercise. Because the thoracic spinal canal is narrow, blood supply and tolerance of spinal cord ischemia is be poor, and severe neurological damage can be easily produced when compressed. Therefore, patients with symptomatic TDH should undergo early surgical treatment [19].
Currently, the clinical surgical methods towards TDH include the anterolateral approach and the posterior approach. The anterolateral approach can be divided into the transthoracic approach and the extrapleural approach. The anterior transthoracic approach has the advantage of an unobscured field, which is convenient for compete decompression, especially for central type or merged disc calcification. However, this approach cannot remove the compression materials under direct vision, so the risk of injuring nerves is still high. At the same time, complications such as pneumonia, pneumothorax, atelectasis, pleural infection, chylothorax and vascular injury have also been reported [20]. Thoracoscopic discectomy has been developed over recent years. This is a new technology used for T4~11 discectomy which also enables intravertebral bone grafting under endoscopy; it has exhibited the possibility of gradually replacing traditional open chest surgery. However, this method requires advanced technology and equipment, causing difficulties in promoting it and increasing its popularity [21,22].
Simple posterior laminectomy was invented in the 1930s. This involves removing the lesion-adjacent vertebral segments and expanding the thoracic spinal sagittal diameter, thus indirectly relieving pressures on the nerves. However, because this surgery failed to directly remove the compression materials, combined with the limited posterior movement of the thoracic spinal cord, the postoperative results were unsatisfactory. In this study, a posterolateral approach was chosen. Through partially resecting the rear lamina and articular process, this approach could fully reveal the herniated compression object from the posterolateral side. Because of the complete direct vision, this maximally avoids interfering with the spinal nerve. This approach was simple, and para-central or soft herniation might be decompressed by unilateral surgery; or, for central or hard herniation we could select bilateral surgery. Theoretically, once a surgeon has mastered transforaminal lumbar interbody fusion or posterior lumbar interbody fusion techniques, it should be easy to implement and carry out this surgery. In addition, where intra-segmental collapse, narrowing and kyphosis are present, intravertebral grafting and segmental stabilization could enable reconstruction after decompression, thus restoring segmental height and reducing local kyphosis. Mulier et al. [23] compared the transthoracic anterior approach and the posterior approach, and believed that while there was no significant difference in efficacy between these two methods, the anterolateral approach had relatively high complication rates and increased trauma, while the posterior approach could not only remove disc oppression, but also effectively remove the combined ossification of the ligamentum flavum.
Complications and prevention
Post-TDH complications include perioperative nerve damage, cerebrospinal fluid leakage, and epidural hematoma, as well as middle- and long-term bone fusion delay or non-fusion, internal fixation loosening, fracture, and recurrence. Nerve damage is the most serious complication, because most TDH is hard herniation, which might exhibit severe symptoms, and most were accompanied with severe nerve damage. Careful exposure and delicate operation is the key to reducing spinal cord and nerve damage. When resecting the disc, the herniated disc might be partially resected through the posterolateral approach; then, the nerve dissector could be used to break into the spinal intervertebral disk and carefully separate it from the endorachis, after which it could be removed from the posterolateral side or pushed forwards in order to avoid injuring the spinal cord. Cerebrospinal fluid leakage is a common complication. In addition to accidental intraoperative tearing, this is mainly seen in the cases combined with disc calcification, ossification of yellow ligament and posterior longitudinal ligament, or those closely adhered to the dura mater. When the dural defect is small, it can be sutured with a non-traumatic suture, and covered with autologous fat or muscle tissues; when the dural defect is large, it can be sprayed with biological glue and covered with gelatin sponge or artificial dura. After surgery, the patient should be under strict bed rest, and if a large amount of fluid is drained, lumbar subarachnoid catheterization for cerebrospinal fluid drainage should be performed. In this study, 3 cases of CSF leakage were treated with the above method and cured. Adequate hemostasis and maintaining unobstructed drainage are keys to avoiding epidural hematoma, and close observation should be undertaken towards the patient’s lower extremity sensations and movements within 24 h after surgery. Once abnormalities in a patient’s sensation and movements are found, emergency treatment should be carried out. Delayed bone grafting fusion or non-fusion are closely related with internal fixation loosening and fracture. Less bone grafting, insufficient bed rest, and lacking rigid internal fixation were the major factors leading to the above phenomena. This study used interbody bone grafting fusion: after completely removing the cartilage endplate, the soft tissue-removed autologous bone particles were then implanted, or tamped then implanted with the intervertebral fusion device, which showed achievement of bone fusion 6-9 months later. No internal fixation loosening or fracture, etc., appeared during follow-up.
Conclusion
Thoracic disc resection is the only effective method for treating TDH. Posterolateral discectomy and interbody fusion surgery is an effective treatment for this disease, but because of the anatomical specificities of the thoracic spinal cord and the thoracic segmental spine, TDH is often associated with ossification and calcification of ligaments, which means that the surgical risks are very large. The surgical operations require the surgeon to be as detailed and precise as possible, to perform a complete decompression whilst avoiding iatrogenic nerve damage and other complications.
Conflicts of Interest
All of the authors declare that they have no conflicts of interest regarding this paper.
References
- Lyu RK, Chang HS, Tang LM, Chen ST. Thoracic disc herniation mimicking acute lumbar disc disease. Spine (Phila Pa 1976) 1999; 24: 416-418.
- Guo JJ, Luk KD, Karppinen J, Yang H, Cheung KM. Prevalence, distribution, and morphology of ossification of the ligamentum flavum: a population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine 2010; 35: 51-56.
- Fernandez M, Gidvani SN. Thoracic disc herniation. Berlin Heidelberg: Springer 2014; 193-201.
- Gao L, Wang L, Su B, Wang P, Ye J. The vascular supply to the spinal cord and its relationship to anterior spine surgical approaches. Spine J 2013; 13: 966-973.
- Klezl Z, Swamy GN, Vyskocil T, Kryl J, Stulik J. Incidence of vascular complications arising from anterior spinal surgery in the thoraco-lumbar spine. Asian Spine J 2014; 8: 59-63.
- Anand N, Regan JJ. Video-assisted thoracoscopic surgery for thoracic disc disease: classification and outcome study of 100 consecutive cases with a 2-year minimum follow-up period. Spine 2002; 27: 871-879.
- Bransford R, Zhang F, Bellabarba C, Konodi M, Chapman JR. Early experience treating thoracic disc herniations using a modified transfacet pedicle-sparing decompression and fusion: clinical article. J Neurosurg Spine 2010; 12: 221-231.
- Lidar Z, Lifshutz J, Bhattacharjee S, Kurpad SN, Maiman DJ. Minimally invasive, extracavitary approach for thoracic disc herniation: technical report and preliminary results. Spine J 2006; 6: 157-163.
- Stillerman CB, Chen TC, Couldwell WT, Zhang W, Weiss MH. Experience in the surgical management of 82 symptomatic herniated thoracic discs and review of the literature. J Neurosurg 1998; 88: 623-633.
- Taher F, Lebl DR, Cammisa FP, Pinter DW, Sun DY, Girardi FP. Transient neurological deficit following midthoracic decompression for severe stenosis: a series of three cases. Eur Spine J 2013; 22: 2057-2061.
- Yoshihara H. Surgical treatment for thoracic disc herniation: an update. Spine (Phila Pa 1976) 2014; 39: 406-412.
- Hur H, Lee JK, Lee JH, Kim JH, Kim SH. Thoracic myelopathy caused by ossification of the ligamentum flavum. J Korean Neurosurg Soc 2009; 46: 189-194.
- Sato T, Kokubun S, Tanaka Y, Ishii Y. Thoracic myelopathy in the Japanese: epidemiological and clinical observations on the cases in Miyagi prefecture. Tohoku J Exp Med 1998; 184: 1-11.
- Otani K, Yoshida M, Fujii E, Nakai S, Shibasaki K. Thoracic disc herniation. Surgical treatment in 23 patients. Spine (Phila Pa 1976) 1988; 13: 1262-1267.
- Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976) 1995; 20: 1410-1418.
- Rohde RS, Kang JD. Thoracic disc herniation presenting with chronic nausea and abdominal pain. A case report. J Bone Joint Surg Am 2004; 86-86A: 379-81.
- Ross JS, Perez-Reyes N, Masaryk TJ, Bohlman H, Modic MT. Thoracic disk herniation: MR imaging. Radiology 1987; 165: 511-515.
- Skubic JW. Thoracic disk diseas. Curr Opin Orthop 1993; 4: 96-98.
- Hanai K, Ogikubo O, Miyashita T. Anterior decompression for myelopathy resulting from thoracic ossification of the posterior longitudinal ligament. Spine 2002; 27: 1070-1076.
- Wait SD, Fox DJ, Kenny KJ, Dickman CA. Thoracoscopic resection of symptomatic herniated thoracic discs: clinical results in 121 patients. Spine 2012; 37: 35-40.
- Benzel EC, Perry TG. Minimally invasive thoracic microendoscopic diskectomy. World Neurosurg 2013; 80: 319-321.
- Yamasaki R, Okuda S, Maeno T, Haku T, Iwasaki M. Surgical outcomes of posterior thoracic interbody fusion for thoracic disc herniations. Eur Spine J 2013; 22: 2496-2503.
- Mulier S, Debois V. Thoracic disc herniations: transthoracic, lateral, or posterolateral approach? A review. Surg Neurol 1998; 49: 599-606.