Case Report - Otolaryngology Online Journal (2016) Volume 6, Issue 3
Posterior Septal Mass Causing Nasal Obstruction
- *Corresponding Author:
- Emily S Tignor
Department of Otolaryngology Head and Neck Surgery, University of Texas Medical Branch Galveston (UTMB), 301 Univeristy Blvd, Galveston, TX-77555, Texas, USA
Tel: 864-567-1603
E-mail: estignor@utmb.edu
Received date: June 11, 2016; Accepted date: July 13, 2016; Published date: July 18, 2016
Abstract
A midline posterior septal mass is a rare finding with a differential diagnosis including benign versus malignant tumors. We present a rare and interesting case of midline posterior septal mass found to be esthesioneuroblastoma on final pathology. We will discuss esthesioneuroblastoma in detail including diagnostic steps, characteristic radiologic findings, grading and staging systems, and treatment.
Keywords
Esthesioneuroblastoma; Septum mass; Nasal obstruction
Case Report
A 77 year old female patient presented with a 1 month history of nasal obstruction, congestion, and left facial pressure. Patient denied epistaxis or sinusitis. Endoscopic nasal exam showed large inferior posterior septal mass that was smooth and present bilaterally.
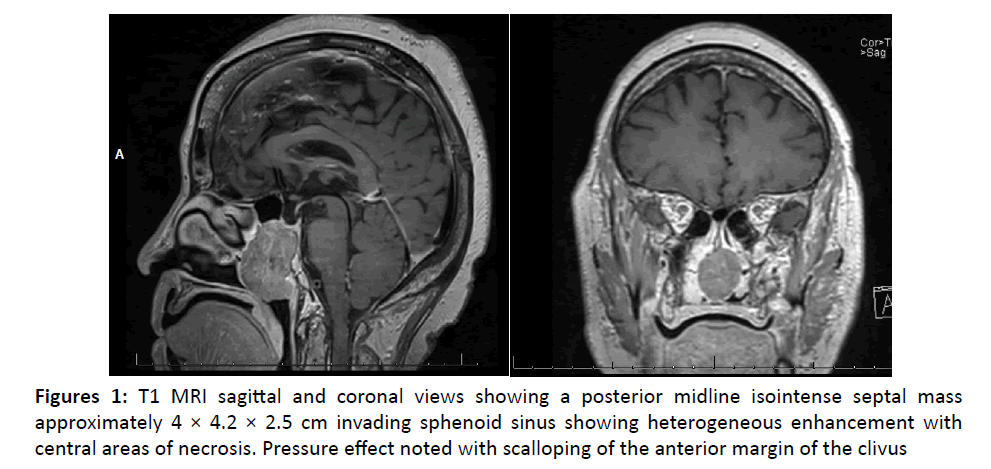
Radiologic studies were performed including CT landmark sinus without contrast and MRI with gadolinium contrast. Imaging showed a posterior midline septal mass invading sphenoid sinus but not extending intracranially (Figure 1).
Discussion
Midline posterior septal masses are uncommon; the differential diagnoses include benign tumors (inverting papilloma, pituitary adenoma, dermoid cyst, encephalocele, polyps, sarcoidosis) and malignant tumors (rhabdomyosarcoma, lymphoma, melanoma, sinonasal undifferentiated carcinoma, sarcoma, chondrosarcoma, squamous cell carcinoma, adenocarcinoma, adenoid cystic carcinoma, and esthesioneuroblastoma). Knowing the radiologic features of these differentials can impact treatment and diagnostic work up.
Esthesioneuroblastoma is a rare cause of midline posterior septal mass in adults with an incidence of 3% of all intranasal tumors [1]. Symptoms of a nasal mass include epistaxis, pain, facial pressure, and nasal obstruction- the most common presenting symptom [2]. Tumor growth is slow and a 6-12 month delay diagnosis after onset of symptoms is common. Lymph node metastasis is rare at initial presentation (5-10%) [3].
This tumor of neuroectodermal origin likely arises from basal neural cells of the olfactory epithelium (also known as olfactory neuroblastoma) and is usually found in the superior nasal vault (cribiform plate, sphenoid sinus, superior nasal septum) [2,4,5]. Rare cases have been reported involving the inferior meatus, maxillary sinus, and one case involving the posterior nasal septum [5]. This tumor is locally invasive often involving skull base or orbit [1]. Our case is unusual due to the inferior posterior nasal septum location.
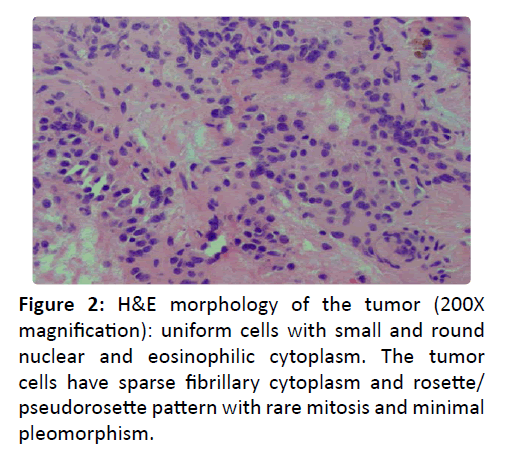
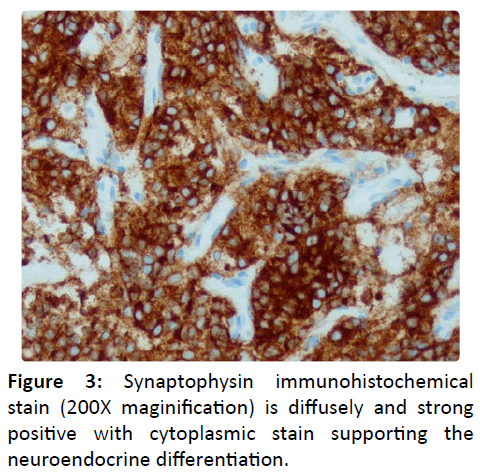
Workup of a nasal mass involves direct visualization, imaging studies, and biopsy. Endoscopic findings are of a submucosal polypoid mass [1]. Contrast CT provides osseous and angiographic detail as well as 3D reconstruction possibilities. MRI illustrates soft tissue anatomy while providing details about nerve infiltration and other soft tissue invasion. Esthesioneuroblastoma is difficult to distinguish from other nasal tumors with imaging alone, but can show peripheral cystic components along the intracranial border. Biopsy shows uniform cells with sparse fibrillary cytoplasm and rosette/ pseudorosette pattern (Figure 2) with tumor cells diffusely and strong positive for synaptophyysin [2] stain (200X maginification) is diffusely and strong positive with cytoplasmic stain supporting the neuroendocrine differentiation.
(Figure 3). Skeletal muscle markers are negative, differentiating the tumor from rhabdomyosarcoma; S-100 is only peripherally positive which highlights the presence of sustentacular cells, differentiating the tumor from melanoma [2].
Hyams grading system is used to classify low (grade 1,2) and high (grade 3,4) grade lesions and takes mitotic activity, architecture, and presence of necrosis into consideration [7]. Multiple staging systems exist including Kadish, Morita, Dulguerov, and Biller [2]. Staging systems measure tumor extent, metastasis, and respectability of tumor. Kadish staging is the most common system utilized and designates stage A as tumor limited to nasal cavity to stage D tumor as invasion of surrounding structures with lymph node or distant metastasis.
Treatment of esthesioneuroblastoma normally involves a combination of modalities including surgery, radiation, and chemotherapy [2,5,6]. Surgery alone may be used for small tumors. However, radiotherapy alone is considered suboptimal [7]. One review indicated 10-15 year survival rates at 76% overall and another found disease specific survival at 80% for 10 years [8-10]. Endoscopic resection has been shown to have low rates of complications and high survival. Other authors recommend postoperative neck dissections for nodal disease and claim that the role of chemotherapy remains questionable [10]. Survival outcomes are greatly affected by the histological grade of the lesion [7]. Low grade lesions (Hyams 1,2) have a 89% 10 year survival while high grade lesions (Hyams 3,4) have a 31% 10 year survival. The addition of radiotherapy does not affect outcomes in high grade lesions [7].
Esthesioneuroblastoma is a disease that requires long term follows up in all patients due to recurrence of disease more than 10 years post treatment [3]. Local recurrence has been reported at 17% for all patients. Treatment modality may affect this and the addition of radiation decreases local recurrence rate. However, late development of lymph node metastasis presents in 18-33% of patients.
The patient in this case report was found to have a stage Kadish B Hyams grade 2 esthesioneuroblastoma and underwent endoscopic resection followed by chemotherapy and radiation therapy. During the surgery, it was noted that the mass was centered on the posterior nasal septum and invaded into the sphenoid sinus, clivus, left sphenopalatine foramen, and left medial pterygoid plate. The patient is currently six months status post resection and doing well without signs of recurrence.
References
- Lee J, Kim H (2007) Primary olfactory neuroblastoma originating from the inferior meatus of the nasal cavity. Am J of Otolaryngol28:196-200.
- Ow T, Bell D, Kupferman M (2013) Esthesioneuroblastoma. NeurosurgClin N Am 24:51-65.
- Bak M, Wein R (2012) Esthesioneuroblastoma: a contemporary review of diagnosis and management. Hematology Oncology Clinics of North America 26:1185-1207.
- Dulguerov P, Allai A, Calcaterra T (2001) Esthesioneuroblastoma: a meta-analysis and review. Lancet 2:683-689.
- Cho K, Lee D, Choi K, Roh H (2010) Primary olfactory neuroblastoma originating from the posterior nasal septum. JAMA Otolaryngol Head Neck Surg142:776-777.
- Chaudhri A, Parmar H, Morales R (2012) Lesions of the skull base: imaging for diagnosis and treatment. OtolaryngolClin N Am 45: 1385-1404.
- Kane A, Sughrue M, Rutkowski M (2010) Posttreatment prognosis of patients with esthesioneuroblastoma. J Neurosurg113:340-351.
- Constantinidis J, Steinhart H, Koch M (2004) olfactory neuroblastoma: the University of Erlangen-Nuremberg experience 1973-2000. JAMA Otolaryngol Head Neck Surg130:567-574.
- Zafereo M, Fakhri S, Prayson R (2008) Esthesioneuroblastoma: 25-year experience at a single institution. JAMA Otolaryngol Head Neck Surg 138:452-458.
- Papacharalampous G, Vlastarakos P, Chrysovergis A (2013) Olfactory neuroblastoma (esthesioneuroblastoma): towards minimally invasive surgery and multi-modality treatment strategies – an updated critical review of the current literature. J BUON 18:2241-6293.