Research Article - Biomedical Research (2018) Volume 29, Issue 13
Possible involvement of cyclooxygenase-2 in the recurrence of desmoid fibromatosis: case series and mini-review of the literature
Kazuhiko Hashimoto*, Shunji Nishimura, Naohiro Oka, Ryosuke Kakinoki and Masao Akagi
Department of Orthopaedic Surgery, Kindai University Hospital, Osaka-Sayama City, Osaka, Japan
- *Corresponding Author:
- Mbamalu ON
Discipline of Pharmacology, School of Pharmacy University of the Western Cape Bellville 7535, South Africa
Tel: +27219593229/2190
Fax: +278615107002
E-mail: ombamalu@uwc.ac.za
Accepted on June 02, 2018
DOI: 10.4066/biomedicalresearch.29-18-733
Visit for more related articles at Biomedical ResearchAbstract
Background: Desmoid fibromatosis (DF) is a type of fibrous tumor that rarely metastasizes but often recurs locally. Cyclooxygenase-2 (COX-2) has been shown to be expressed in DF lesions, although its involvement in DF recurrence is unknown. We investigated the association between COX-2 expression and DF recurrence using immunohistochemistry.
Patients and Methods: Cases of DF treated in our hospital between March 2000 and December 2012 were reviewed. We used immunohistochemistry to identify COX-2 expression in resected specimens from these cases. The relationship between recurrence and age, sex, duration of disease, size of tumor, tumor site, spontaneous pain, inflammation, and COX-2 expression was studied.
Results: In the ten included cases (mean age, 55.2 y; 5 male and 5 female patients), we found that COX-2 expression was significantly associated with DF recurrence (p=0.01).
Conclusion: Our findings indicate that COX-2 is involved in recurrence of DF.
Keywords
Desmoid-type fibromatosis (DF), Cyclooxygenase-2 (COX-2), Recurrence, immunohistochemical analysis.
Introduction
Desmoid fibromatosis (DF) is a locally invasive, relatively rare mesenchymal neoplasm that develops from the connective tissue, fascia, and aponeurosis of skeletal muscle [1]. Although it is non-metastatic, morbidity due to local complications and the local recurrence rate after resection is high, ranging from 20%-40% [2]; It arises in numerous locations, but most often in the limbs, waist, scapula, and the trunk, including the abdominal cavity [3].
DF accounts for approximately 0.03% of all soft tissue tumors [4]. In sporadically arising cases, approximately 7.5% of DF patients have a history of Familial Adenomatous Polyposis (FAP) and have an 800-fold increased risk of developing DF compared to the general population [5]. DF affects a wide range of ages, but it is most common among those between 10 and 40 y of age [1-3]. In the paediatric population, it arises equally in both sexes, and is predominantly abdominal [2,3]. Between puberty and 40 y old, tumors are most common in women, and predominantly arise in the abdominal wall [6,7]. Later in adulthood, the distribution between abdominal and extra-peritoneal tumors becomes more even, and the sex ratio of affected patients tends towards parity [1,5-7].
The etiology of DF is multifactorial, with endocrine, physical and genetic elements thought to be involved [8]. Several factors indicate the role of estrogen in its development, including the high incidence of pregnant women who develop the condition, and disease regression or stabilization after estrogen blockade [8]. Several studies have demonstrated estrogen receptor (ERβ) positivity in up to 90% of neoplasms [2,9,10]. DF has also been reported to occur at sites of previous surgery or blunt trauma [2,11,12]. Sporadic cases show a mutation in the β-catenin gene on 3p21, resulting in overexpression of β-catenin in the nucleus [13,14]. 14β-catenin plays an important role in regulating the size of skin wounds and mediates the action of TGF-β, which is known to promote wound hyperplasia through proliferation of fibroblasts [13,14]. Thus, it can be assumed that DF represents partially uncontrolled wound healing [2].
Histologically, DF is usually invasive and consists of uniform myofibroblast sweep capsules in a high density collagen stroma [3]. Approximately 80% of neoplasms show granular nuclear expression of β-catenin [13,14]. Histologic differential diagnosis is broad and encompasses a wide range of spindle cell lesions, ranging from scarring to abdominal gastrointestinal stromal tumors and liposarcoma with ‘low grade’ dedifferentiation [15,16]. However, most neoplasms resembling fibroma have specific histological diagnostic characteristics and can be confidently diagnosed by the absence of nuclear β-catenin immunoreactivity [13,14].
Due to the unpredictable natural history of the disease, it is difficult to establish a treatment regimen for DF [17]. Historically, surgery was the main treatment with or without radiation therapy [2,17]. Recently, some studies have reported spontaneous regression and delayed illness in cases that are not receiving treatment, and many institutions and investigators have therefore proposed a ‘wait and see’ monitoring policy [18,19]. This strategy enables the identification of patients who remain asymptomatic with stable illness or experience spontaneous regression and protects this cohort from unnecessary treatment.
Amongst these findings, there has been evidence that cyclooxygenase-2 (COX-2) is involved in DF pathogenesis. Some previous reports have shown that COX-2 inhibitors were effective to treat intra-abdominal DF and that COX-2 inhibitors reduce local progression of DF [18,20]. However, to date there have been no reports investigating the role of COX-2 in DF recurrence. Therefore, we conducted this study to investigate whether COX-2 is involved in DF recurrence.
Materials and Methods
Ten cases of DF who underwent surgical treatment with a wide margin at our hospital from March 2000 to December 2012 were reviewed. COX-2 immunostaining was performed on excised pathological specimens from each patient using rabbit monoclonal COX-2 antibody (1/100 dilution, GeneTex; GTX16701). We investigated the relationship between DF recurrence and various patient characteristics, including age, sex, duration of disease, tumor size, site of the mass (abdominal or extra-abdominal), presence of spontaneous pain, presence of inflammatory response, and COX-2 expression. We analyzed these factors using the chi-squared test. Since anonymity was maintained, it was not necessary to obtain informed consent from patients. This research was approved by the Ethics Committee of Kindai University Faculty of Medicine.
Results
In total, 10 patients with DF were included in this study. The mean age was 55.2 y (range, 24-83 y), and there were 5 male and 5 female patients. COX-2 expression was positive in 5 of the 10 patients; of these, all 5 developed DF recurrence. Of the remaining 5 patients in which COX-2 expression was negative, none had DF recurrence. Statistical analysis identified a significant association between COX-2 expression and DF recurrence (p=0.01). The other patient characteristics, including age, sex, duration of disease, site of the mass, size, presence of pain, and presence of an inflammatory response, were not associated with DF recurrence.
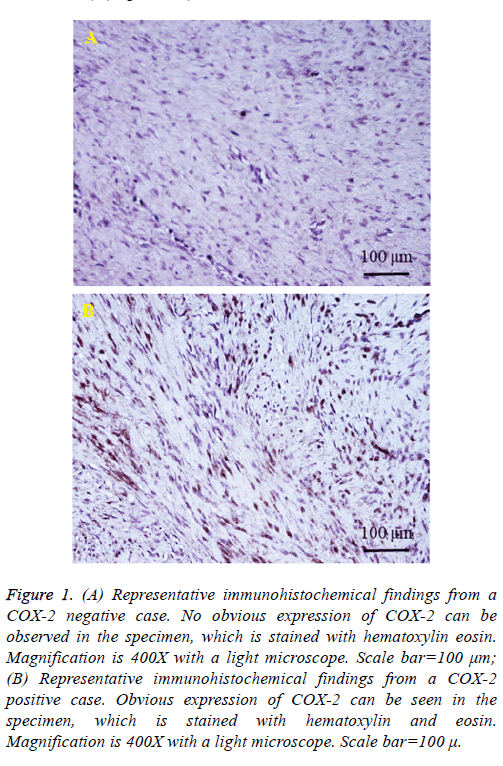
We present two representative example cases. The first case involves a 56-y-old man who presented with left forearm pain as a chief complaint. He noticed a lump in the left forearm 4 months prior to presenting at our hospital, but only visited the hospital when the site became painful. The tumor size was 3 × 4 cm. A blood test revealed a C-reactive protein level of 0.013 (mg/dl). The tumor mass underwent resection with wide margins, and no recurrence was observed in the 2 y after surgery. Expression of COX-2 in the excised specimens was negative (<10 COX-2-positive cells/total tumor cells) (Figure 1A).
The second case features a 77-y-old man whose chief complaint was the presence of a soft tissue mass in the left elbow. He recognized a tumor in the left elbow 1 y ago at his first hospital visit. Due to the gradual increase in size, the patient visited his doctor, and was referred to our department. The tumor size was measured to be 4 × 6 cm. He recognized no spontaneous pain. A blood test revealed a C-reactive protein level of 0.014 (mg/dl). We resected the tumor mass with wide margins; the patient remained recurrence-free for 1 y after surgery. Expression of COX-2 in the excised specimen was positive (COX-2 positive: ≥ 10 COX-2-positive cells/total tumor cells) (Figure 1B).
Figure 1: (A) Representative immunohistochemical findings from a COX-2 negative case. No obvious expression of COX-2 can be observed in the specimen, which is stained with hematoxylin eosin. Magnification is 400X with a light microscope. Scale bar=100 μm; (B) Representative immunohistochemical findings from a COX-2 positive case. Obvious expression of COX-2 can be seen in the specimen, which is stained with hematoxylin and eosin. Magnification is 400X with a light microscope. Scale bar=100 μ.
| Case no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean ± SD or n/n | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 83 | 24 | 61 | 37 | 57 | 37 | 90 | 56 | 30 | 77 | 55.2 ± 21.3 | 0.65 |
| Sex | Female | Male | Female | Female | Male | Female | Male | Male | Female | Male | M5/F5 | 0.88 |
| Duration of disease | 5 y | 6 y | 1 y | 3 months | 2 months | 3 y | 1 y | 4 months | 8 y | 1 y | 16.6 ± 23.3 | 0.67 |
| Site | Scapula | Sacrum | Neck | Abdomen | Left upper arm | Gluteal | Right forearm | Left upper arm | Retroperitoneal | Left elbow | 2 intra-abdominal, 8 extra-abdominal | 0.98 |
| Size (cm) | 5 × 8 | 3 × 6 | 3 × 8 | 3 × 4 | 3 × 3 | 6 × 13 | 4 × 6 | 1.5 × 1.5 | 5×8 | 6×8 | ± | 0.54 |
| Pain | - | - | - | - | - | + | + | + | + | - | -6/+4 | 0.73 |
| Inflammation in blood | - | - | - | - | - | + | - | - | - | - | -9/+1 | 0.61 |
| COX-2 expression | + | + | - | - | - | + | + | + | - | - | 5/5 | 0.01 |
| Recurrence | + | + | - | - | - | + | + | + | - | - | 5/5 | 0.01 |
Table 1. Summary of the ten cases included in the study.
Discussion
DF is known to have a high incidence of local recurrence [2,13]. Recurrence is often problematic, as there are no clear treatments indicated for DF recurrence [17]. We conducted an investigation into factors involved in DF recurrence using immunohistochemistry and discovered that COX-2 expression was significantly associated with recurrence. This suggests that COX-2 may be involved in the recurrence of DF.
Previous studies have shown that COX-2 is involved in DF pathogenesis and that it can alter the progression of DF [18,20]. The mutated form of the β-catenin gene (CTNNB1) has also been suggested as a factor that can influence the cellular activity of DF [13,14]. One of the roles of COX-2 is suppression of β-catenin gene expression [13]. In the current study, COX-2 expression was significantly associated with the cases with recurrence, suggesting that COX-2 is in some way involved in the DF recurrence mechanism.
Recent reports have suggested that DF should be treated using a ‘wait and see’ policy [18,19] in which oncologists should monitor the tumor prior to treatment with medication, such as a COX-2 inhibitor, or resection. Interestingly, a previous case report showed that pancreatic DF was cured using a COX-2 inhibitor [21]. Moreover, a previous pilot study also showed that COX-2 inhibitor treatment for intra-abdominal DF was effective [18]. These findings suggest that COX-2 inhibitors might be a suitable option for treatment of DF. Combined with the results from the present study, we suggest that COX-2 inhibitor would be effective for recurrence case of DF.
In conclusion, we examined whether COX-2 was associated with recurrence of DF. We found that positive expression of COX-2 was significantly associated with DF recurrence. We suggest that COX-2 positive cells should be the treatment target in future cases of DF recurrence.
Acknowledgement
We would like to thank Editage (www.editage.jp) for English language editing.
References
- Martin D, Muradbegovic M, Andrejevic-Blant S, Petermann D, Di Mare L. Omental fibromatosis treated by laparoscopic wide surgical resection. Intractable Rare Dis Res 2018; 7: 51-53.
- Awe O, Eluehike S. Desmoid fibromatosis of the lower abdominal wall in Irrua. Nigeria. Niger J Surg 2018; 24: 52.
- Liu X, Zong S, Cui Y, Yue Y. Misdiagnosis of aggressive fibromatosis of the abdominal wall. Medicine (Baltimore) 2018; 97: e9925.
- Sinno H, Zadeh T. Desmoid tumors of the pediatric mandible: case report and review. Ann Plast Surg 2009; 62: 213-219.
- Nieuwenhuis MH, Casparie M, Mathus-Vliegen LMH, Dekkers OM, Hogendoorn PCW, Vasen HFA. A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer 2010; 129: 256-261.
- Wirth L, Klein A, Baur-Melnyk A, Knösel T, Lindner LH, Roeder F, Jansson V, Dürr HR. Desmoid tumours of the extremity and trunk. A retrospective study of 44 patients. BMC Musculoskelet Disord 2018; 19: 2.
- Szurian K, Till H, Amerstorfer E, Hinteregger N, Mischinger H-J, Liegl-Atzwanger B, Brcic I. Rarity among benign gastric tumors: Plexiform fibromyxoma-Report of two cases. World J Gastroenterol 2017; 23: 5817.
- Mignemi NA, Itani DM, Fasig JH, Keedy VL, Hande KR, Whited BW, Homlar KC, Correa H, Coffin CM, Black JO, Yi Y, Halpern JL, Holt GE, Schwartz HS, Schoenecker JG, Cates JM. Signal transduction pathway analysis in desmoid-type fibromatosis: Transforming growth factor-β, COX2 and sex steroid receptors. Cancer Sci 2012; 103: 2173-2180.
- Carter JM, Howe BM, Hawse JR, Giannini C, Spinner RJ, and Fritchie KJ. CTNNB1 mutations and estrogen receptor expression in neuromuscular choristoma and its associated fibromatosis. Am J Surg Pathol 2016; 40: 1368-1374.
- Quast DR, Schneider R, Burdzik E, Hoppe S, Möslein G. Long-term outcome of sporadic and FAP-associated desmoid tumors treated with high-dose selective estrogen receptor modulators and sulindac: a single-center long-term observational study in 134 patients. Fam Cancer 2015; 15: 31-40.
- Nakanishi K, Shida D, Tsukamoto S, Ochiai H, Mazaki J, Taniguchi H, Kanemitsu Y. Multiple rapidly growing desmoid tumors that were difficult to distinguish from recurrence of rectal cancer. World J Surg Oncol 2017; 15: 180.
- Campos FG, Martinez CAR, Novaes M, Nahas SC, Cecconello I. Desmoid tumors: clinical features and outcome of an unpredictable and challenging manifestation of familial adenomatous polyposis. Fam Cancer 2014; 14: 211-219.
- Hamada S, Urakawa H, Kozawa E, Arai E, Ikuta K, Sakai T, Ishiguro N, Nishida Y. Characteristics of cultured desmoid cells with different CTNNB1 mutation status. Cancer Med 2015; 5: 352-360.
- Hamada S, Urakawa H, Kozawa E, Futamura N, Ikuta K, Shimoyama Y, Nakamura S, Ishiguro N, Nishida Y. Nuclear expression of β-catenin predicts the efficacy of meloxicam treatment for patients with sporadic desmoid tumors. Tumor Biol 2014; 35: 4561-4566.
- Magro G. Differential diagnosis of benign spindle cell lesions. Surg Pathol Clin 2018; 11: 91-121.
- Munakomi S. Aggressive fibromatosis in the infratemporal fossa presenting as trismus: a case report. J Med Case Rep 2018; 12: 41.
- Calvert GT, Monument MJ, Burt RW, Jones KB, Randall RL. Extra-abdominal desmoid tumors associated with familial adenomatous polyposis. Sarcoma 2012; 2012: 1-11.
- Nishida Y, Tsukushi S, Shido Y, Wasa J, Ishiguro N, Yamada Y. Successful treatment with meloxicam, a cyclooxygenase-2 inhibitor, of patients with extra-abdominal desmoid tumors: a pilot study. J Clin Oncol 2010; 28: 107-109.
- Penel N, Chibon F, Salas S. Adult desmoid tumors. Curr Opin Oncol 2017; 29: 268-274.
- Poon R, Smits R, Li C, Jagmohan-Changur S, Kong M, Cheon S, Yu C, Fodde R, Alman BA. Cyclooxygenase-two (COX-2) modulates proliferation in aggressive fibromatosis (desmoid tumor). Oncogene 2001; 20: 451-460.
- Wang Y-C, Wong J-U. Complete remission of pancreatic head desmoid tumor treated by COX-2 inhibitor-a case report. World J Surg Oncol 2016; 14: 190.