Case Report - Current Trends in Cardiology (2021) Volume 5, Issue 2
Platypnea-Orthodeoxia syndrome in a patient with atrial septal defect and concomitant aortic root enlargement.
Eustaquio Maria Onorato1*, Francesco Casilli2, Gian Paolo Anzola3
1Department of Cardiology, Centro Cardiologico Monzino, IRCCS University School of Milan, Milan, Italy
2Department of Cardiology, Centro di Cardiologia Interventistica, Istituto Clinico Sant’Ambrogio, Via Luigi Giuseppe Faravelli, Milan, Italy
3Department of Neurology, PFH Parma, Milan, Italy
- Corresponding Author:
- Eustaquio Maria Onorato
Department of Cardiology
Centro Cardiologico Monzino
IRCCS University School of Milan
Milan, Italy
E-mail: eustaquio.onorato@gmail.com
Accepted date: 22 January, 2021
Citation: Onorato EM, Casilli F. Platypnea-Orthodeoxia syndrome in a patient with atrial septal defect and concomitant aortic root enlargement. Curr Trend Cardiol. 2021; 5(2):31-34.
Abstract
We present the case of hypertensive and dislipidemic adult patient with a small ostium secundum atrial septal defect with trivial left-to-right shunt in whom an increasing enlargement of the aortic root and ascending aorta reversed the interatrial shunt causing severe dyspnea and oxygen desaturation, constantly elicited during upright position and relieved when he resumed the recumbent position (Platypnea-Orthodeoxia syndrome). Catheter-based closure of the interatrial communication was associated with an immediate increase in systemic saturation and long lasting marked clinical improvement.
Keywords
Platypnea-Orthodeoxia syndrome, Atrial septal defect, Percutaneous interventions, Echocardiography, Transcranial doppler.
Introduction
Ostium Secundum Atrial Septal Defect (ASD II) with a leftright shunt accounts for 10% of all congenital heart disease and as much as 20%-40% of those presenting in adulthood. It can be asymptomatic through infancy and childhood, though the timing of clinical presentation depends on the degree of left-to-right shunt. By the age of 40 years, 90% of untreated patients have symptoms of exertional dyspnea, fatigue, palpitation, sustained arrhythmia or even evidence of heart failure.
We present the case of an otherwise asymptomatic adult patient with ASD II and mute left-to-right shunt in whom an increasing enlargement of the aortic root and ascending aorta reversed the interatrial shunt causing life-threatening dyspnea with deoxygenation in the absence of elevated right heart pressures.
Case Report
A 53 years old man, suffering from hypertension, dyslipidemia, coronary artery disease (previous lateral myocardial infarction) and aortic root enlargement with moderate aortic incompetence, underwent left radical nephrectomy for renal carcinoma incidentally discovered by a computed tomography. After surgical operation, he had persistent microcytic anemia: a retroperitoneal hematoma due to spleen rupture was discovered and splenectomy was successfully performed. Few months later he was re-admitted again to hospital because he was noted to have profound respiratory distress and oxygen desaturation to less than 88% when sitting up on room air. The functional respiratory tests did not reveal obstructive and/or restrictive lung disease. Chest computed tomography, spirometry, and lung perfusion scintigraphy showed no findings of lung disease or pulmonary embolism. Oxygen desaturation and dyspnea were constantly elicited during upright position and relieved when he resumed the recumbent position (O2 saturation 66% vs. 94%). This behaviour strongly resembles that occurring in the platypneaorthodeoxia syndrome (POS).
In order to ascertain this hypothesis, the patient was referred to our institution. At admission the electrocardiogram showed synus rhythm, 85 beats/min, incomplete right bundle branch block and delayed QRS transition zone in the precordial leads in addition to low voltage (Figure 1).
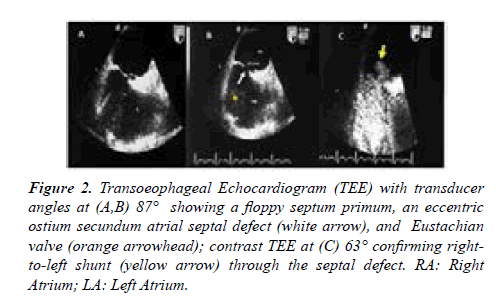
Chest X ray demonstrated aortic elongation and a systolic murmur was detected at the left sternal border. The main laboratory tests revealed mild renal dysfunction (creatinine 2.4 mg/dl, urea 40 mg/dL); the other parameters were within normal range. Signs of deconditioning with muscular legs weakening were present due to prolonged bed rest. Two-dimensional Transthoracic Echocardiography (TTE) revealed ASD II with bidirectional shunt, normal pulmonary venous drainage, significant enlargement of the aortic root (58 mm) and ascending aorta (42 mm) with associated moderate aortic incompetence, normal left ventricular volumes and systolic function. Transoeophageal Echocardiography (TEE) with transducer angles at 87° showed a small septal defect (white arrow), 13 m3 in diameter, a floppy septum primum and an Eustachian valve; contrast TEE at 63° confirmed a right-to-left shunt (RLS) (yellow arrow) through the septal defect (Figures 2A-2C).
Figure 2:Transoeophageal Echocardiogram (TEE) with transducer angles at (A,B) 87° showing a floppy septum primum, an eccentric ostium secundum atrial septal defect (white arrow), and Eustachian valve (orange arrowhead); contrast TEE at (C) 63° confirming right-to-left shunt (yellow arrow) through the septal defect. RA: Right Atrium; LA: Left Atrium.
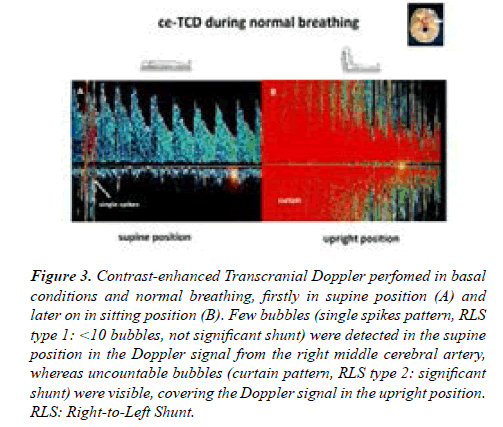
Contrast-enhanced TransCranial Dopper (ce-TCD) was performed according to a standardized procedure [1]. 10 mL of air-mixed saline was injected into the right antecubital vein while the Doppler signal from the right middle cerebral artery was recorded. Less than 10 bubbles were detected in the supine position (single spikes), whereas uncountable bubbles (curtain pattern) were present in the upright position confirming the presence of a large significant posture-dependent RLS (Figures 3A and 3B).
Figure 3:Contrast-enhanced Transcranial Doppler perfomed in basal conditions and normal breathing, firstly in supine position (A) and later on in sitting position (B). Few bubbles (single spikes pattern, RLS type 1: <10 bubbles, not significant shunt) were detected in the supine position in the Doppler signal from the right middle cerebral artery, whereas uncountable bubbles (curtain pattern, RLS type 2: significant shunt) were visible, covering the Doppler signal in the upright position. RLS: Right-to-Left Shunt.
In consideration of the significant shunt obtained already in basal conditions, no further provocative tests (Valsalva maneuver) were performed. Right heart catheterization confirmed interatrial communication with trivial left-to-right shunt and pulmonary to systemic flow ratio (Qp/Qs) of 1.09/1. Pulmonary artery pressure was in the normal range (30-10-20 mmmHg) as well as right-sided pressures (Table 1).
| O2 Saturations | |
|---|---|
| SVC | 73% |
| IVC | 80% |
| RA | 80% |
| PA | 76% |
| LA | 98% |
| Pressures | |
| PA | 30-10-20 mmmHg |
| RV | 40-0/12 mmHg |
| RA | 14 mmHg(mean) |
| Arota | 130-80-97 mmmHg |
Abbreviations: SVC: Superior Vena Cava; IVC: Inferior Vena Cava; PA: Pulmonary Arota; RV: Right Ventricular; B02: Oxygen Carrying Capacity of Heamoglobin
Table 1. Baseline resting hemodynamic measurements and oxygen saturations for the assessment of pulmonary-to-systemic blood flow ratio (Qp/Qs).
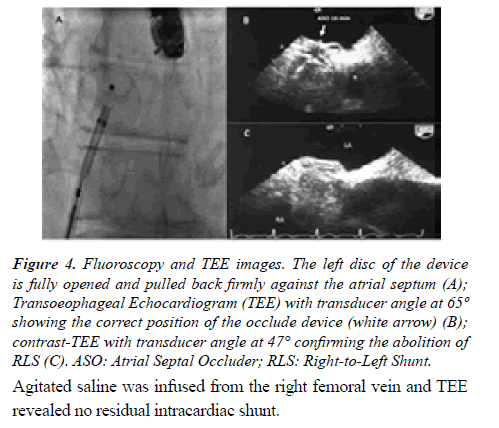
After heart team discussion, it was decided to address his congenital heart disease using a catheter-based technique. An informed consent was signed by the patient. The procedure was performed in the catheterization laboratory under deep sedation and continuous real time 2D/3D TEE color flow Doppler and fluoroscopic guidance. The interatrial comunication was crossed under fluoroscopy with a 6 Fr multipurpose catheter. After placing a standard 0.0035-in- 260 cm exchange wire in the upper left pulmonary vein, stretched diameter by balloon sizing was obtained (15 mm). Afterwards, a 10 Fr transeptal sheath was advanced over the exchange wire into the left atrium. An Amplatzer Atrial Septal Device (16 mm) device was chosen. The left-sided disc was pulled back firmly against the atrial septum, followed by deployment of the right-sided disc. At the end of the procedure and before the release of the device, the position of the occluder was checked both by fluoroscopy and by TEE (Figures 4A-4C).
Figure 4:Fluoroscopy and TEE images. The left disc of the device is fully opened and pulled back firmly against the atrial septum (A); Transoeophageal Echocardiogram (TEE) with transducer angle at 65º showing the correct position of the occlude device (white arrow) (B); contrast-TEE with transducer angle at 47° confirming the abolition of RLS (C). ASO: Atrial Septal Occluder; RLS: Right-to-Left Shunt.
Agitated saline was infused from the right femoral vein and TEE revealed no residual intracardiac shunt.
Post-procedure blood gas analysis normalized even in the sitting position and symptoms disappeared completely. He was discharged home in good clinical conditions. Persistent clinical improvement and no changes in arterial oxygenation or RLS in upright position were confirmed at the follow-up.
Discussion
POS, firstly described by Burchell et al. is an uncommon cause of dyspnea characterised by breathlessness that is exacerbated when the patient is in the upright position (“platypnea”) and arterial oxygen desaturation that is worsened when the patient is in the upright position (“orthodeoxia”). Notably, RLS occurs despite normal right atrial pressure and vascular pulmonary resistance, may be elicited by sitting or by abdominal compression and can be extremely debilitating from a functional point of view [1,2].
The incidence of POS is unknown and the literature is limited to case reports. Nevertheless, increasing articles over the past years have elicited the attention of physicians who have acquired a greater awareness of this syndrome.
The most comprehensive review includes 188 patients from 105 case reports, where 167 patients (89%) were found to have POS due to an intracardiac shunt; when considering those involving an intracardiac RLS with normal pulmonary artery and right atrial pressures, 88.3% had a patent foramen ovale (PFO) [3,4].
The suggested POS pathophysiology is complex and it has puzzled scientists and physicians for decades. Fundamentally, two mechanisms must concur to explain this syndrome. Firstly, an intracardiac anatomical primary defect such as interatrial communication, mainly represented by a PFO and less frequently by a true ASD II or a fenestrated atrial septal aneurysm with/without a secondary anatomical variant such as lipomatous hypertrophy of septum secundum, Chiari network, large Eustachian valve. Secondly, a functional factor which might cause interatrial septum deformation, redirection of shunt flow, stretching of an interatrial defect that occurs with a postural change and finally a decreased right ventricular compliance. Functional factors can be vascular (aortic aneurysm or elongation), pulmonary (pulmonary arteriovenous malformation, pulmonary embolism, idiopathic pulmonary hypertension, severe chronic obstructive pulmonary disease, status/post pneumonectomy), cardiac (constrictive pericarditis, right ventricular infarction, right atrium mixoma), abdominal (liver cirrhosis, diaphragm paralysis, abdominal surgery) and dorsal kyphosis [5,6]. Futhermore, the distorted anatomical arrangement of great veins relative to the atria is assumed to worsen in the upright position.
Notably, aortic root dilation and elongation are very common in the adult population. The dilated ascending aorta is oriented horizontally, compressing the interatrial septum towards the right atrium to lead venous return from the inferior vena cava to the interatrial communication [7-13].
The diagnosis of this syndrome can be challenging because it is largely underdiagnosed due to its specific postural features [14,15]. TTE/TEE allows visualization of atrial septum and provides the opportunity to look for defects, aneurysm or other abnormalities. Nevertheless, the most sensitive and easy to perform modality for non-invasive functional assessment is ce-TCD which probably represents the most suitable tool to quantify RLS with the patient in sitting or upright position, identifying both cardiac and extra cardiac shunts [16]. Of note, testing in the standing position might be warranted when postural variations of RLS can be pathogenically relevant like in POS [17].
The primary anatomical defect in our patient is represented by ASD II associated with an intracardiac shunt but not large enough to generate right volume overload; additionally, the concomitant presence of an associated secondary anatomical variant (Eustachian valve) and two functional factors (aortic root-ascending aorta enlargement and two previous abdominal surgeries) could have certainly increased the interatrial septum distortion/displacement and mobility and foster markedly RLS as it happens in PFO patients. Catheter-based closure of the interatrial communication is a low risk and effective treatment for POS and it is associated with an immediate increase in systemic saturation and symptom improvement [18-20].
Conclusion
This case highlights the importance of considering POS when evaluating unexplained dyspnea and arterial desaturation. Definitive treatment of POS with an identified interatrial communication is surgical or percutaneous closure of the defect, the latter being mostly the first-line preferred method. Ce-TCD represents the most sensitive and reliable modality for non-invasive functional assessment of RLS.
Conflict of Interests
None.
Funding Statement
No sources of funding have supported this Clinical Image.
Author Contributions
Conceptualization and Data Curation: Eustaquio Maria Onorato, Gian Paolo Anzola
Writing-original draft preparation: Eustaquio Maria Onorato, Francesco Casilli
Writing-review and editing: Eustaquio Maria Onorato, Gian Paolo Anzola
The authors have read, edited and reviewed the manuscript.
References
- Jauss M, Zanette E. Detection of right-to-left shunt with ultrasound contrast agent and transcranial doppler sonography. Cerebrovasc Dis. 2000; 10(6):490-6.
- Burchell EH. Wood, reflex orthostatic dyspnea associated with pulmonary hypotension. Am J Physiol. 1949; 159:563-4.
- Rodrigues P, Palma P, Sousa-Pereira L. Platypnea-Orthodeoxia syndrome in review: Defining a new disease? Cardiology. 2012; 123(1):15-23.
- Arias AA, Oberti PF, Falconi ML, et al. Platypnea-Orthyodeoxia syndrome, a hidden cause of dyspnea? Argent J Cardiol. 2012; 80(5):382-4.
- Cheng TO. Platypnea‐orthodeoxia syndrome: Etiology, differential diagnosis, and management. Cathet Cardiovasc Interv. 1999; 47(1):64-6.
- Agrawal A, Palkar A, Talwar A. The multiple dimensions of Platypnea-Orthodeoxia syndrome: A review. Respir Med. 2017; 129:31-8.
- Shiraishi Y, Hakuno D, Isoda K, et al. Platypnea-Orthodeoxia syndrome due to PFO and aortic dilation. JACC Cardiovascular Imaging. 2012; 5(5):570-1.
- Medina A, de Lezo JS, Caballero E, et al. Platypnea-Orthodeoxia due to aortic elongation. Circulation. 2001; 104(6):741.
- Chopard R, Meneveau N. Right-to-left atrial shunting associated with aortic root aneurysm: A case report of a rare cause of Platypnea-Orthodeoxia syndrome. Heart Lung Circ. 2013; 22(1):71-5.
- Akagi S, Taguchi E, Dan K, et al. Right-sided heart failure due to compression of the right atrium by remarkable ascending aortic elongation. Circulation. 2005; 112:e252.
- Hasegawa M, Nagai T, Murakami T, et al. Platypnoea-Orthodeoxia syndrome due to deformation of the patent foramen ovale caused by a dilated ascending aorta: A case report. Eur Heart J Case Rep. 2020; 4(2):1-4.
- Keenan NG, Brochet E, Juliard JM, et al. Aortic root dilatation in young patients with cryptogenic stroke and patent foramen ovale. Arch Cardiovasc Dis. 2012; 105(1):13-7.
- Eicher JC, Bonniaud P, Baudouin N, et al. Hypoxaemia associated with an enlarged aortic root: A new syndrome? Heart. 2005; 91(8):1030-5.
- Tsuzuki I, ligaya K, Matsubara T, et al. Platypnea-Orthodeoxia syndrome in the right lateral decubitus position: A case report. J Med Case Rep. 2017; 11(1):109.
- Klein MR, Kiefer TL, Velazquez EJ. Platypnea-Orthodeoxia syndrome: To shunt or not to shunt, that is the question. Tex Heart Inst J. 2016; 43(3):264-6.
- Anzola GP, Morandi E, Casilli F, et al. Does transcatheter closure of patent foramen ovale really “shut the door?” a prospective study with transcranial doppler. Stroke. 2004; 35(9):2140-4.
- Caputi L, Carriero MR, Parati EA, et al. Postural dependency of right to left shunt. Role of contrast-enhanced transcranial doppler and its potential clinical implications. Stroke. 2008; 39(8):2380-1.
- Guerin P, Lambert V, Godart F, et al. Transcatheter closure of patent foramen ovale in patients with Platypnea-Orthodeoxia: Results of a multicentric French registry. Cardiovasc Intervent Radiol. 2005; 28(2):164–8.
- Henriksen PA, Strachan K, Selby C, et al. Percutaneous patent foramen ovale closure in a patient with Platypnoea-Orthodeoxia syndrome. Heart. 2007; 93(7):892.
- Mojadidi MK, Gevorgyan R, Noureddin N, et al. The effect of patent foramen ovale closure in patients with Platypnea-Orthodeoxia syndrome. Catheter Cardiovasc Interv. 2015; 86(4):701-7.