- Biomedical Research (2009) Volume 20, Issue 1
Plasma adenosine deaminase activity and antioxidant status in preeclam-psia compared to healthy pregnant and nonpregnant women
Rukmini M S1*, Kowsalya R1, Pai B1, Das P1, Perriera J1, Nandini M1 and Asha K21Department of Biochemistry, Kasturba Medical College, Mangalore, Karnataka, India
2Department of Statistics, Kasturba Medical College, Manipal, Karnataka, India
- *Corresponding Author:
- Rukmini.M.S.
Department of Biochemistry,
Centre for Basic Sciences,
Kasturba Medical College,Bejai
Mangalore 575004, Karnataka
India
Accepted date: Ocober 15 2009
Abstract
Preeclampsia is a multisystemic disorder specific to human pregnancy. It is common and major complication, characterized by hypertension, proteinuria and oedema. This clinical condition adversely affects the mother and the foetus. The causative factors remain poorly understood. Oxidative stress is implicated in preeclampsia which could provide the linkage between decreased placental perfusion and the maternal syndrome. Pregnancy has also been associated with depressed cell mediated immunity. The increase in activity of adenosine deaminase (ADA) in preeclamptic women compared to normotensive pregnant women is reported in earlier studies. The main objective of the present study was to correlate the adenosine deaminase activity with the antioxidant status in preeclamptic pa-tients. The study groups aged between 20 – 35 years included 20 normotensive primigravida wom-en and 20 primigravida diagnosed as preeclampsia in their last trimester. The control group included 20 healthy nonpregnant women volunteers of the same age group. The diagnosis of preeclampsia was made by a gynecologist at Lady Goshen Hospital. The adenosine deaminase activity and antioxidant status in the form of superoxide dismutase (SOD), glu-tathione (GSH) and total antioxidant activity (TAA) were measured. The extent of oxidative stress was estimated by measuring plasma malondialdehyde (MDA) levels. The comparison of MDA between controls and preeclamptic patients showed significance (p 0.000). The total antioxidant activity (TAA) between the controls and preeclamptic pa-tients showed significance (p 0.000). However, ADA activity did not show statistical signifi-cance among the different study groups. The ADA activity may not be significant due to other systemic causes such as anemia and malnutrition.
Keywords
Adenosine deaminase, oxidative stress, antioxidants.
Introduction
Preeclampsia occurs in 3% to 5% of pregnancies. It is also a major cause of maternal mortality (15% to 20%) in developed countries and a leading cause of preterm birth and intrauterine growth retardation [1,2].
There is substantial evidence to suggest that the endothe-lial cell injury and altered endothelial cell function may play a role in pathogenesis of preeclampsia [3,4]. The vascular endothelium is the target for the disease process involved in preeclampsia [5]. Maternal vascular endothe-lial dysfunction may be the cause of altered vascular reac-tivity, vasospasm and platelet aggregation [6].
Lipid peroxidation and antioxidant status has been impli-cated in the aetiology of preeclampsia [7,8,9]. Oxidative stress is a component of preeclampsia, which could pro-vide the linkage between decreased placental perfusion and the maternal syndrome [10]. Many reports suggest that lipid peroxidation products, primarily measured as thiobarbituric acid reactive substances which include ma-londialdehyde (MDA) are increased in plasma / sera of women with preeclampsia [7,8].
Increase in plasma adenosine deaminase (ADA) activity in preeclamptic women compared to the normotensive pregnant women has been reported [11].Serum total anti-oxidant activity (TAA), erythrocytic superoxide dismu-tase (SOD) activity and glutathione levels are decreased in women with preeclampsia compared to the normoten-sive pregnant women [12-14]. However, no correlation between ADA activity and antioxidant status has been reported and this will be the main objective of the present study.
Subjects and Methods
The research design included three study groups as fol-lows.
Group I (Control) - 20 healthy nonpregnant women
Group II (Normal Pregnant) - 20 normotensive Primi gra-vida women
Group III (Preeclamptic)- 20 primigravida women with preeclampsia
All the subjects included were aged between 20 - 35 years. The control group comprised of healthy volunteers of the same age group. The subjects admitted to obstetric wards of the Lady Goshen Hospital of Mangalore were selected. The diagnosis of preeclampsia was made by a gynecologist at Lady Goshen Hospital. An informed con-sent was obtained from all the subjects enrolled for the study. The study was approved by the Institutional Hu-man Ethics Committee.
Inclusion criteria: Normotensive primigravida women and preeclamptic primigravida in their last trimester
Exclusion criteria: Multigravida, pregnant women with anemia and gestational DM were excluded.
Sample Collection
Blood was collected from Group II and Group III subjects between 33-36 weeks of pregnancy. 5ml of venous blood was collected randomly from the antecubital vein under aseptic precautions from each subject. 2.5 ml of whole blood was collected in EDTA bottles and 2.5 ml whole blood was collected in plain bottles. The sample was then subjected to centrifugation for 3000g for10 minutes within three hours of collection. Plasma collected care-fully was used for the assay of lipid peroxidation and adenosine deaminase. Serum separated from whole blood collected in plain bottles was used for the assay of total antioxidant activity. Remaining whole blood was mixed with 0.9% saline and centrifuged. Supernatant was re-moved and this process was repeated three times to pre-pare RBC suspension which was used for assay of SOD and reduced glutathione.
Plasma MDA - was measured using the modified method of Satoh [15-17]. Malondialdehyde a secondary product of lipid peroxidation, reacts with thiobarbituric acid (TBA) in acidic medium to give a pink coloured pigment at 97°C at pH 2-3. The pink colour was extracted with butanol and the absorbance read at 535 nm.
Adenosine deaminase - was measured by using Giusti’s method [18]. Ammonia is liberated by the action of adenosine deaminase on adenosine. Ammonia forms an intense blue indophenol with phenol nitroprusside solu-tion and alkaline sodium hypochlorite. The reaction cata-lyzed by ADA is stopped at the end of the incubation pe-riod by the addition of the phenol nitroprusside. The in-tensity of the blue colour is directly proportional to the amount of ammonia liberated and was measured spectro-photometrically at 620nm.
Total antioxidant activity – was measured by the method of Koracevic et al [19]. The assay measured the capacity of the serum to inhibit the production of thiobarbituric acid reactive substances (TBARS) from sodium benzoate under the influence of free oxygen radicals derived from Fenton’s reaction. The pink colour developed was meas-ured spectrophotometrically and the inhibition of color development defined as AOA.
SOD – The method of Beauchamp and Fridovich was followed [20]. Illumination of riboflavin in the presence of O2 and electron donor like methionine or EDTA gener-ates superoxide anions (O2•- ) and this has been the ba-sis for assay of SOD. The reduction of nitrobluetetra-zolium (NBT) by O2•- to blue coloured formazan was followed at 560nm.
Glutathione – was determined by the method of Beutler et al [21]. This is based upon the development of a rela-tively stable yellow color when 5, 5' – dithiobis dinitro benzoic acid is added to sulphydryl compounds. The yel-low color was measured at 412nm.
Statistical analysis
The data was analysed using SPSS software version 15.0 For the comparison of values between the groups, one way analysis of variance was used when the data was normally distributed. Kruskal Wallis test was used for skewed data. For the correlation, Pearsons' correlation coefficient was used.
Results
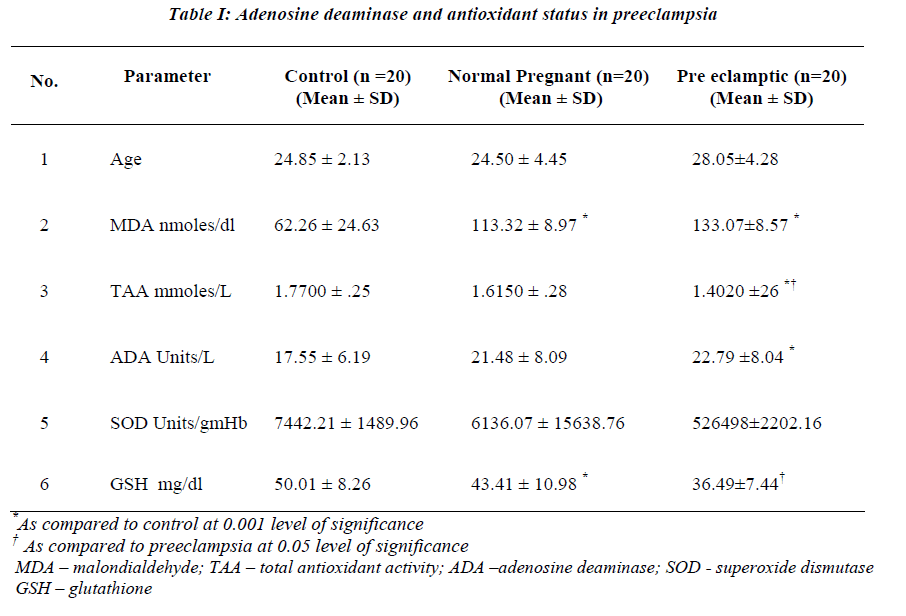
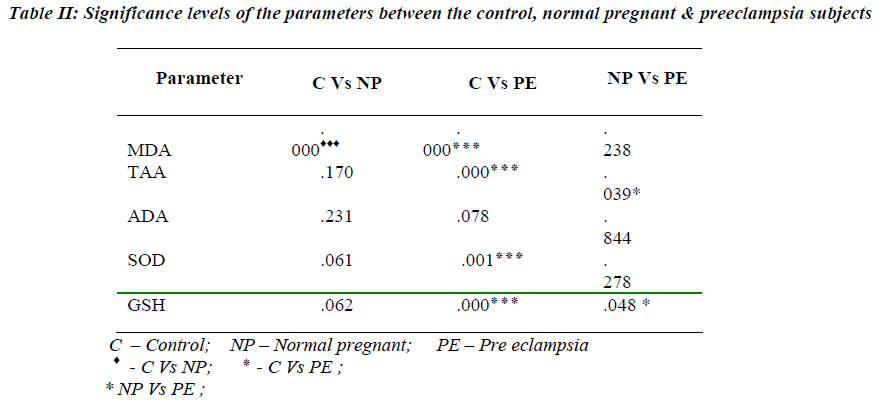
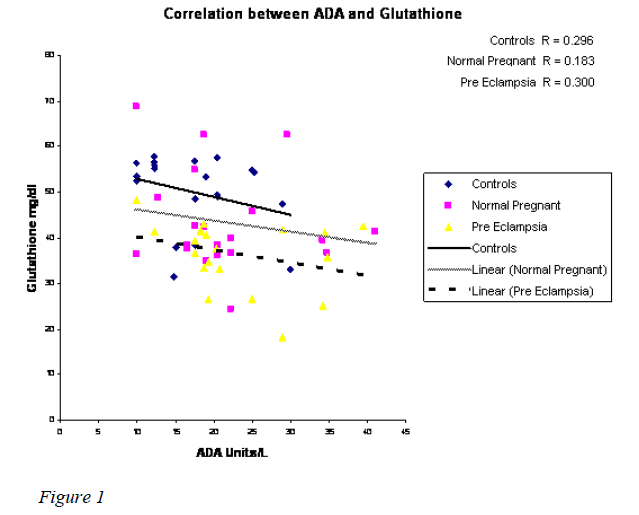
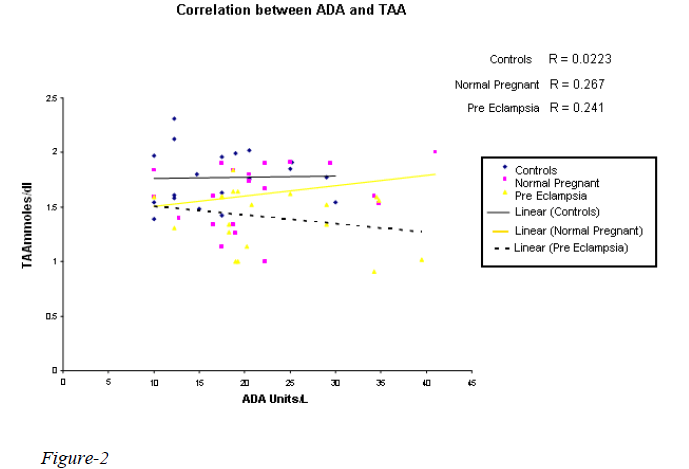
A highly significant increase in MDA value (p 0.000) in normal pregnant & preeclampsia in comparison with the controls was observed with the mean value of 113.32 & 133.07nmoles/dl respectively (Table I). However, there was no statistical significance between the normal preg-nant and preeclampsia. Statistically significant reduction in TAA & GSH was observed in preeclampsia when compared to normal pregnants with the mean value of 1.4020 & 1.6150 mmoles/L and 36.49 & 43.41 mg/dl (Table II) with the p value of 0.039 & p 0.048 respectiv-ely. A significant reduction in SOD (7442.21 Units/gmHb & 5264.98 Units/gmHb), TAA (1.7700 mmoles/L and 1.4020 mmoles/L) and GSH (50.01 mg/dl and 36.49 mg/dl) in preeclampsia was observed when compared to controls. A weakly positive association was observed with ADA Vs glutathione & TAA (Figures 1,2). How-ever, ADA did not show any statistical significance in either of the groups. Also, there was no correlation be-tween plasma adenosine activity and MDA or the antioxi-dant enzyme SOD.
Discussion
Preeclampsia is characterized by endothelial dysfunction and alterations of immune response may be involved in the pathogenesis of this disease. Pregnancy has been as-sociated with depressed cell mediated immunity [22]. Oxidative stress may be an important factor in the patho-genesis of preeclampsia and the antioxidant status in preeclampsia is complex [23].The possible role of cell mediated immunity in preeclampsia has been evaluated by assessing the adenosine deaminase activity [11].
This enzyme is essential for the differentiation of the lymphoid cells and has been used for monitoring several diseases in which immunity is altered [24].
The present study demonstrated a significant increase in plasma MDA in preeclampsia as compared to normal pregnant women. This finding is in agreement with other studies [14,25,26].Yoneyama et al [27] have also reported increased plasma MDA in preeclampsia which was posi-tively correlated to ADA activity. On the other hand, Jer-onimo et al have reported decrease in plasma ADA in normal pregnants (24). On the contrary, the present study reports non significant ADA levels without any correla-tion of MDA with ADA activity suggesting the absence of T-cell activation.
An important finding of the study is the statistically non significant difference in the ADA levels. Several studies have reported increase in plasma ADA in preeclampsia which in turn may be responsible for maintenance of im-mune response [28,29]. Karabulet et al have opined that increased ADA as a marker of immunological disorder may be related to the pathogenesis of the disease [28].
The antioxidant status is reflected by a decrease in RBC SOD and GSH in preeclampsia and normal pregnants. These findings are in line with previous studies [14,30,31,32]. There was no correlation between lipid peroxi-dation and antioxidant enzymes. This is in agreement with the report of Bayhan et al [14].
That oxidative stress is the principal causative factor, is reflected by increase in MDA and decrease in TAA activ-ity. Significant decrease in TAA is observed not only in preeclampsia but also in normal pregnants. A 12.5 fold decrease was observed in preeclampsia when compared to normal pregnancy. This finding is consistent with the report of Davidge et al [33] and Harma et al [34].
Current theory holds that oxidative stress is an imbalance between maternal prooxidants and antioxidants. From the results it can be concluded that impaired antioxidant ac-tivity and the reduction of antioxidants could be the pos-sible cause for the increased lipid peroxidation observed which may cause damage to vascular endothelium result-ing in clinical symptoms of preeclampsia. Administration of antioxidants in early pregnancy may help in preventing the complication resulting in preeclampsia and may be a fruitful therapeutic strategy. One of the limitation of the study was the assessment of the antioxidant status was done only during last trimester and not followed at the time of delivery. Further studies on the mechanism of ADA regulation may provide information on its added role in the pathogenesis of preeclampsia.
Acknowledgements
The authors thank the Obstetrician and Gynecologist Dr. Bharathi Nirmal, patients, and the staff at Lady Goshen Hospital, Mangalore for their help and support.
References
- Hubel CA. Oxidative Stress in the Pathogenesis of Preeclampsia. Proc. Soc. Exp.Biol.Med Review. 1999; 222 (3): 222-235.
- Working Group report on high blood pressure in pregnancy: Consensus report. Working Group on High Blood Pressure in Pregnancy. National High Blood Pressure Education Program. Am J Obstet.Gynecol. 1990; 163: 1689-1712.
- Rappaport VJ, Hirata G, Yap HK, Jordon SC. Antivas- cular endothelial cell antibodies in severe preeclampsia. Am J Obstet Gynecol 1990; 162: 138-146.
- Rodgers GM, Taylor RN, Roberts JM. Preeclampsia associated with a serum factor cytotoxic to human en- dothelial cells. Am J. Obstet Gynecol 1988; 159: 908-914.
- Roberts JM. Objective evidence of endothelial dysfunc- tion in preeclampsia. Am. J. Kidney Disease 1999; 33: 992-997.
- Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, Mc Laughlin MK. Preeclampsia: An endothelial cell disorder Am. J. Obstet. Gynecol. 1990; 163: 1365-1366.
- Walsh SC. Lipid peroxidation in pregnancy. Hyperten- sion Pregnancy 1994; 13:1-25.
- Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM, Mc Laughlin MK. Lipid peroxidation in pregnancy: New perspectives on preeclampsia. Am. J. Ob- stet. Gynecol. 1989; 161: 1025-1034.
- Gratacos E, Casals E, Deulofeu R, Gomez O, Casarach V, Alano P, Fortury A. Serum antibodies to oxidized low- density lipoprotein in pregnant women with pree- clampsia and chronic hypertension, lack of correlation with lipid peroxides. Hypertens. Pregnancy 2001; 20, 170-183.
- Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of preeclampsia? Lancet 1999; 353: 788-789.
- Yoneyama Y et al. Serum adenosine deaminase activity in women with preeclampsia. Gynecologic & Obstetric Investigation 2002; 54: 164-167.
- Sagol S, Ozkinay E, Ozsraser S. Impaired antioxidant activity in women with preeclampsia. International Journal of Gynecology and Obstetrics 1999; 64: 121-127.
- Kharb S. Total free radical trapping antioxidant poten- tial in preeclampsia. International Journal of Gynecol- ogy & Obstetrics 2000; 69: 23-26.
- Bayhan G, Atamer Y, Atamer A, Yokus B, Baylan Y. Significance of changes in lipid peroxides and antioxi- dant enzyme activities in pregnant women with pree- clampsia and eclampsia. Clin Exp Obstet Gynecol 2000; 27: 142-146.
- Satoh K. Serum lipid peroxide in Cerebrovascular diso- rders determined by a new colorimetric method. Clinica Chem Acta.1978; 90: 37-43.
- Poornima K, Cariappa M, Asha K, Kedilaya HP, Nandini M. Oxidant and Antioxidant status in Vege- tarians and Fish eaters. Ind J. Clin. Biochem. 2003; 18: 197-205.
- Wasowicz W, Neve J, Pereitz A. Optimized steps in fluorometric determination of thiobarbituric acid reac- tive substances in serum: Importance of extraction, pH and influences of sample preservation and storage. Clin Chem 1993; 39: 2522 -2526.
- Giusti G. Adenosine deaminase. In: Bergneyer HU, Methods of enzymatic analysis. 2nd Edition. New York: Academic Press Inc. 1974; 1092- 1098.
- D Karacevic, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurements of antioxidant activity in human fluids. J Clin Pathol 2001; 54: 356- 361.
- McCord, Fridovich IM. Superoxide dismutase on en- zymatic function for erythrocumein. J Biol.Chem 1969; 24: 6049- 6055.
- Beutler et al. Improved method for the determination of blood glutathione. J Lab and Clinical Med 1963; 61: 883- 887.
- Weinberg ED. Pregnancy- associated depression of cell mediated immunity. Rev Infect Dis 1984; 6: 814- 831.
- Zusterzeel PL et al. Ethene and other biomarkers of oxidative stress in hypertensive disorders of pregnancy. Hypertens Pregnancy 2002; 21: 243-246.
- Jeronimo Jaqueti et al. Adenosine Deaminase in Preg- nancy Serum. Clin.Chem. 1990; Vol 3 6, No 12, 2144.
- Muthlu-Turkoglu U et al. Imbalance between Lipid Peroxidation and Antioxidant Status in Preeclampsia. Gynecol Obstet Invest. 1998; 46: 37- 40.
- Dekker GA, Sibai BM. The Immunology of Preeclam- psia. Seminars in Perinatology 1999; 23: 24-33.
- Yoneyama et al. Relationship between plasma malondi- aldehyde levels and adenosine deaminase activities in preeclampsia. Clin Chim Acta 2002; 322: 169 -173.
- Karabulut AB, Kafkasli A, Burak F, Gozukara EM. Maternal and fetal adenosine deaminase, xanthine oxi- dase and malondialdehyde levels in preeclampsia. Cell Biochem Funct 2004; 28, 23: 279-283.
- Yoneyama et al. Relation between adenosine deami- nase activities and cytokine- producing T cells in women with preeclampsia. Clin Biochem 2002; 35: 303-306.
- Alexa ID, Jerca L. The role of oxidative stress in the aetiology of preeclampsia: changes at the GSH and GSH-Px levels in normal pregnancy and preeclampsia. Rev Med Chir Soc Med Nat Iasi. 1996; 100: 131-135.
- Poranen AK, Ekblad U, Uotila P, Ahotupa M. Lipid peroxidation and antioxidants in normal and pree- clamptic pregnancies. Placenta 1996; 17: 401-405.
- Aydin S, Benian A, Madazli R, Uludag S, Uzun H, Kaya S. Plasma malondialdehyde, superoxide dismu- tase, selectin, fibronectin, endothelin -1 and nitric oxide levels in women with preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004; 113: 21-25.
- Davidge et al, Sera antioxidant activity in uncompli- cated and preeclamptic pregnancies. Obstet. Gynecol. 1992; 79: 897-901.
- Harma M, Harma M, Erel O. Measurement of the total antioxidant response in preeclampsia with a novel automated method. Eur J Obstet Gynecol Reprod Biol 2005; 118: 47-51.