- Biomedical Research (2015) Volume 26, Issue 4
Plant synthesis of silver nanoparticles from Matricaria chamomilla plant and evaluation of the antibacterial and antifungal effects
Masoud Negahdary1, Shahin Omidi2, Ali Eghbali-Zarch1, Seyed Abdolhossein Mousavi3, Gholamreza Mohseni4, Yazdan Moradpour5 Ghasem Rahimi6*
1Yazd Cardiovascular Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
2Department of Biology, Islamic Azad University, Arsanjan Branch, Arsanjan, Iran
3Department of Health Services Management, Bushehr University of Medical Sciences, Bushehr, Iran
4Department of Anesthetics, Shahid Beheshti University of Medical Sciences, Tehran, Iran
5Department of Plant Pathology, Marvdasht branch, Islamic Azad University, Marvdasht, Iran
6Young Researchers and Elite Club, Marvdasht Branch, Islamic Azad University, Marvdasht, Iran
- Corresponding Author:
- Ghasem Rahimi
Young Researchers and Elite Club
Marvdasht Branch
Islamic Azad University
Marvdasht, Iran
Accepted date: July 21 2014
Abstract
One of the outstanding goals in nanotechnology, is produce the nanoparticles with the ability to control and reduce costs and environmental pollution. Recently, nanoparticles produced using living biological systems to create a new branch of nanotechnology. Silver nanoparticles are one of the most widely used nanoparticles. This study aimed to produce silver nanoparticles with Matricaria chamomilla and evaluation antibacterial and antifungal effects was performed, first Matric aria chamomilla extract were prepared by boiling, then in the vicinity of the extract with 20mM silver nitrate salt, reaction in the dark and at ambient tempura was carried out. Physicochemical properties of nanoparticles produced using a spectrophotometer and SEM device was studied. Eventually, the antibacterial and antifungal effects of these nanoparticles was evaluated. After study of the spectrum of UV-Vis and SEM showed that Matricaria chamomilla capable of producing silver nanoparticles extracellular with rang of 60 to 65nm and with is different morphology. It is clear that the nanoparticles have good antibacterial and antifungal properties against bacteria and yeast that used in this study. Matricaria chamomilla is a reducing agent suitable and compatible with environment for produce nanoparticles.
Keywords
Plant synthesis of nanoparticles, Matricaria chamomilla, silver nanoparticles, antibacterial effects, antifungal effects
Introduction
Nanotechnology is an interdisciplinary field and is formed from the convergence of chemistry, physics and biology. We see unique physicochemical properties when the size of the particles is reduced. In fact, with entering the realm of nanotechnology, we see that surface area to volume ratio and quantum behavior are the most important characteristics that are seen during the shrinking of the size of particles [1]. Because of the low toxicity to cells in the body and high surface plasmon resonance, silver nanoparticles have numerous applications in diagnosis and treatment of diseases, particularly cancer, use in catalysts and biosensors and elimination of bacterial and fungal infectious agents[2]. Achieving nanoparticles with the ability to control size and shape and synthesis of nanoparticles that have desirable properties and are compatible with the environment are among the goals of nanotechnology. So far, different methods have been defined for the synthesis of nanoparticles in the field of nanotechnology. Each of these methods has advantages and disadvantages depending on the reactions used. Currently nanoparticles are commercially synthesized by chemical method. These methods have resulted in the creation of a production attitude that is appropriate and compatible with the environment due to environmental pollutions resulted from the material used in chemical synthesis such as reducing agents for reducing metal ions and stabilizing agents for preventing the accumulation of nanoparticles that are used in various processes, low yields due to excessive consumption of energy to produce nanoparticles, high cost of production and the low quality of nanoparticles produced due to high temperatures [3, 4]. Recently, biosynthesis of nanoparticles using biological systems such as bacteria, fungi, yeasts and plants has been defined as a new branch of nanotechnology due to the high effectiveness and efficiency and being compatible with the environment [5,6]. Nanoparticle production using plants can be useful for different purposes considering the ability to control the size and dispersion level of particles. The production of nanoparticles using plant extracts has been proved by many researchers [7]. This study was conducted with the purpose of plant synthesis of silver nanoparticles using Matricaria chamomilla and assessment of its antibacterial and anti-fungal effects.
Materials and Methods
All materials and equipment used in this study including sabouraud dextrose agar media, sabouraud dextrose broth media, Nutrient broth media, Meuller- Hinton agar media, and silver nitrate for the synthesis of nanoparticles were purchased from Merck company in Germany. Standard strain of the yeast candida albicans (ATCC 14053) and staphylococcus aurous (ATCC 25923) were purchased from the Center of Scientific and Industrial Research of Iran.
Production of Silver nanoparticles from Matricaria chamomilla
In this research silver nanoparticles were synthesized from Matricaria chamomilla extract. Boiling method was used to prepare extract of dried Matricaria chamomilla. First, 25 gr of dried Matricaria chamomilla was placed in an Erlenmeyer flask and its volume was brought to 100 ml using deionized water. Erlenmeyer flask contents were boiled for 5 minutes on flame. The extract obtained using this method was filtered using filter paper and 0.45 μm microfilters after cooling. Then 20 mM silver nitrate was added to the extract. The lid of the container containing extract and silver nitrate was closed and the container was kept in a dark place for reaction [8].
Exploring physic-chemical characteristics of nanoparticles
UV-visible spectroscopy
In order to explore the UV-visible characteristics of silver nanoparticles synthetized from Matricaria chamomilla 200 μm of the solution resulted from reaction of the extract of the aforementioned plant and silver nitrate was brought to the volume of 1 ml in cuvette and the spectroscopy was done using spectrophotometer device (at wavelengths of 400-800 nm). Silver nitrate solution with Matricaria chamomilla extract at zero time was used as the control group [8].
Electron microscopic study of silver nanoparticles
For electron microscopic study of nanoparticles produced from Matricaria chamomilla, sediment resulted from the reaction of the plant extract and silver nitrate was centrifuged for 5 minutes using a centrifuge device at the speed of 12000 rpm. The sediment resulted in this stage was used to investigate the electron microscope [8].
Exploring the antibacterial effect of the produced nanoparticles
For exploring the antibacterial effects of the produced nanoparticles the effects of these nanoparticles was explored on staphylococcus aureus ATCC (25923) in NB media culture. To evaluate the effect of nanoparticles in liquid media, 100 μl of a suspension of nanoparticles with concentration of 0.37×108 Particles ml-1 in NB medium containing 1×107 cellml-1bacterial suspension was inoculated. The tube containing suspension of silver nanoparticles and bacteria was incubated at 37 ° C for 48 h. To assess the effects of these nanoparticles, the optical density (OD) was measured in spectrophotometer at wavelength of 600 nm and 0, 2, 4, 6, 8, 10, 12, 24,48 times and its diagram was drawn. The media containing bacterial suspension without any nanoparticles were used as control group [9, 10].
Exploring the antifungal effect of the produced nanoparticles
To evaluate the antifungal effects of nanoparticles synthesized from Matricaria chamomilla the effect of nanoparticles on biofilm formation in pathogenic yeast Candida albicans (ATCC 14053) was evaluated. The biofilm of this yeast was formed in 99-well micro plate. After pouring 100μl of fungal suspension containing 1 × 106 cell in each milliliter into wells of microplate, the microplate was placed in a shaking incubator at 37 ° C with 75 rpm in order for the cells to attach to the bottom of the well. To isolate unattached from buffer, phosphate buffered saline with pH 7.4 was used in a way that each well was washed three times with 100 μl of phosphate buffered saline with pH 7.4. Then 100 μl of medium (SDB) was added to all wells and microplate was kept in a shaking incubator at 37° C for 48 h.
After this time, the formed biofilm was washed again with 100 μl of phosphate buffered saline. Then 100 μl of suspension of biosynthesized silver nanoparticles with concentration of 0.37 ×108 Particles ml-1 and 100 μl of medium were added to the wells and incubated for 24 h. The amount of the formed biofilm was measured using MTT assay. Thus, 50 μl of MTT solution was mixed with with 50 μl cell suspension and 50 μl of medium and the mixture was kept at 37 ° C for 24 h.
After washing the wells with phosphate buffered saline 100 μl of dimethyl sulfoxide solution was added to the wells and the wells were kept at 37o C for 15 minutes. Finally, the optical absorption of micro plates was measured with ELISA reader at a wavelength of 490 nm. At the same time, microscopic observations were done at the presence of the concentration 0.37 ×10 8 Particles ml-1.
For this purpose, the silver nanoparticles with concentration of 0.37×108 Particles ml-1 were mixed with yeast cells and the effects of nanoparticles at 0 and 24 hours were examined using a stereo microscope. For control group the yeast cells without any nanoparticles were used [11].
Results
Spectrophotometric analysis
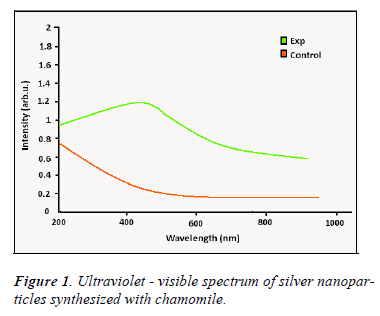
Figure 1 shows the ultraviolet- visible spectrum of nanoparticles synthesized using Matricaria chamomilla. According to this figure an absorption peak is created in the range of 200 -800 nm which indicates a surface Plasmon vibration at wavelength of 420-450 nm compared to the control group and verifies the special and quantum properties of nanoparticles synthesized by Matricaria chamomilla. This spectroscopy is consistent with the findings of other researches in which silver nanoparticles were synthesized using plant.
Electron microscopic study of silver nanoparticles
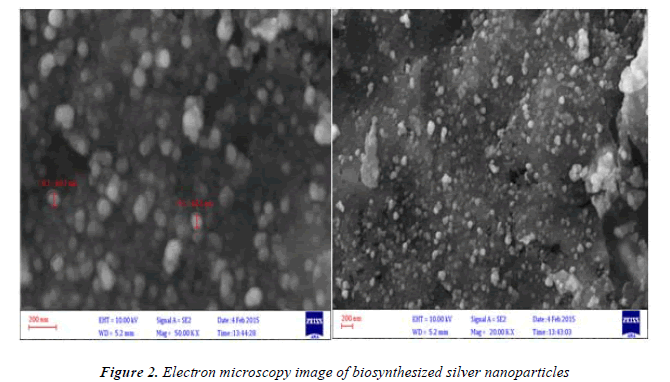
Figure 2. Shows electron microscopy image of silver nanoparticles synthesized with Matricaria chamomilla. As it can be seen in the figure synthesized nanoparticles have dimensions of 60-65 nm and are in different forms which are synthesized in the form of extracellular synthesis. Since the nanoparticles are synthesized in extracellular way, reduction of costs of obtaining nanoparticles in this method a very important advantage compared with chemical synthesis of nanoparticles. Data from electron microscopy shows that the nanoparticles are produced in different sizes and this is one of the limitations of producing nanoparticles by method. However, by controlling the experimental conditions, including changes in PH and temperature and controlling the used doses of reactants, such as reducing solution (silver nitrate) and reductant (extract), this limitation can be overcome.
Exploring the antibacterial effect of the produced nanoparticles
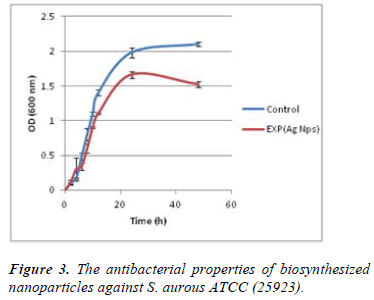
Figure 3 shows the effects of silver nanoparticles against bacteria Staphylococcus aureus in broth medium. As shown in Figure 3, optical absorption of bacterial suspension containing biosynthesized silver nanoparticles during 0 to 48 hour time at the presence of nanoparticles is significantly reduced compared with the control group.
Exploring the antifungal effect of nanoparticles produced
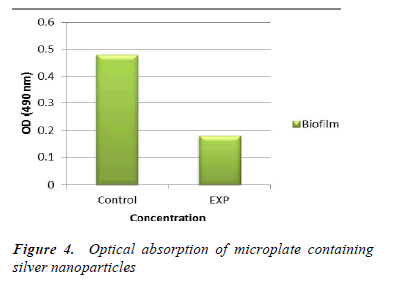
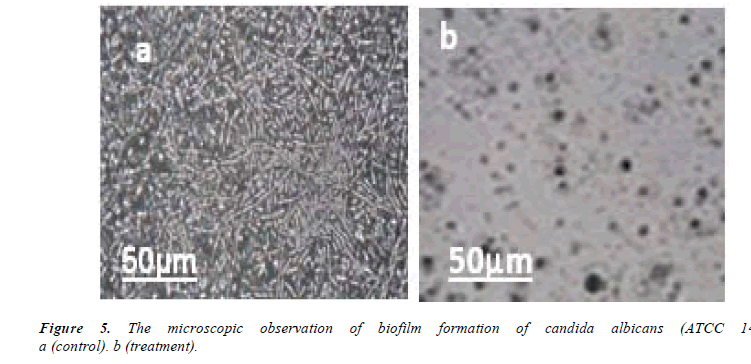
In the study of antifungal effect of synthesized nanoparticles it was revealed that these nanoparticles have favorable effects on biofilm formation in pathogenic yeast Candida albicans. Figure 4 shows optical absorption of microplate at 490 nm wavelength. As it is evident in the picture, silver nanoparticles have reduced optical absorption of microplate compared with the control group. This effect is shown in Figure 5. As it is evident in the picture, nanoparticles synthesized from Matricaria chamomilla result in a marked reduction in the biofilm of the yeast compared with the control group. After 24 hours of treatment of yeast cells with synthesized silver nanoparticles the density and size of the cells were reduced.
Discussion
Toxic effects of nanoparticles produced using chemical method are a serious threat to human health and a potential risk to the environment. Nanoparticles produced with biological and environment-friendly methods, can be prevent the potential risks of chemical methods on humans and the environment [12]. Production of nanoparticles using live biological systems such as microorganisms (bacteria and fungi) and plants has been reported by many researchers. So far, most of the studies on the production of nanoparticles in the world have been done using bacteria and fungi [13]. In one study it was shown that Lactobacillus strains at exposure of silver nitrate solution result in the creation of silver nanoparticles in periplasmic space of the bacteria [14]. In one study it was concluded that Verticillium can result in the reduction of silver nitrate and production of silver nanoparticles with the size of 5 to 50 nm [15]. However, the production of nanoparticles by plants, especially medicinal plant is one of the methods that, in addition to the advantages over chemical methods, result in the reduction of risk and prevention of infection by microorganisms. In addition, the synthesis of nanoparticles using plants will be advantageous over other methods in terms of compatibility with the environment. In this study, production of silver nanoparticles using Matricaria chamomilla was evaluated for the first time. The results obtained in this study are consistent with other researches on production of silver nanoparticles by plants [16].
According to reports, UV-Vis spectra recorded for biosynthesized silver nanoparticles are in the range of 400- 450 nm. In this research too, the results of UV-Vis spectra indicate the increase of surface plasmon vibrations at 420 - 450 nm wavelength. Scanning Electron Microscopy data indicate that silver nanoparticles size range is from 60 to 65 nm and with different morphologies. The results in this study are consistent with other studies [17, 18]). Shankar and et al synthesized silver nanoparticles from Pelargonium leaves. Their results showed that the plant is able to produce nanoparticles with dimensions of 16-40 nm [19]. In another study, Karimi and Mohsen Zadeh synthesized silver nanoparticles from Achillea millefolium. Their research showed that Achillea millefolium is able to synthesize silver nanoparticles with dimensions of 39-226 nm [8]. Compared to the aforementioned study, our data showed that Matricaria chamomilla synthesize silver nanoparticles in the size range of 60-65 nm. Silver nanoparticles have wide applications including being antibacterial and anti-fungal agents, as a catalyst in chemical reactions and biological, use in electrical batteries, use in nonlinear optics and etc. [20]. Extracellular production of nanoparticles is one of the essential and important parameters in biosynthesis of nanoparticles as in this way achieving nanoparticles are easy and costs are lower for their purification [21]. Production of silver nanoparticles using plants results in the reduction of costs of obtaining nanoparticles. The biosynthesized nanoparticles, like chemically-synthesized nanoparticles, have the specific physicochemical properties needed for use in medical and industrial purposes and compared to with chemicallysynthesized nanoparticles, biosynthesized nanoparticles have been highly paid attention to in recent years due to ease of production, repeatability, high volumes of production and the lack of production of pollution and waste. In this study it was tried to produce silver nanoparticles in extracellular form with the aim of creating optimal physicochemical conditions compared with chemical methods. Matricaria chamomilla is a suitable and environmentfriendly reducing agent for the production of nanoparticles and this study can pave the way for industrial production of silver nanoparticles by Matricaria chamomilla and other plants in the future.
References
- Buzea C, Blandino I, Robbie K .Nanomaterial andnanoparticles: Sources and toxicity. J.Bio interphases 2007; 2: 172-80.
- Brains KC, D’Souza, S. F. Development of a preliminary. Biosynthesis of silver nanoparticles using the fungus Aspergillums fumigates.J. BI 2006; 47(4):160- 164.
- Mandala D, Bolander ME, Mukhopadhyay D, SarkarG, Mukherjee P. The use of microorganisms for the formation of metal nanoparticles and their application.Appl. Microbiol. J.Bioethanol 2006; 69: 458- 492.
- Nersisyan HH, Lee JH, Son HT, Won CW, Maeng DY. A new and effective chemical reduction method for preparation of Nano sized silver powder and colloid dispersion. J.Mater Res Bull 2003; 38: 949-956.
- Guru, S, Kalishwaralal K, Vaidya R, Venkataraman D, Pandian S, Muniyandi J, et al. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. J.Colloids and Surfaces Bio interfaces 2009; 74: 328-335.
- Hernández-Sierra JF, Ruiz F, Cruz D, Martinez- Gutiérrez F, Martinez AE, Guillen A, et al. The antimicrobial sensitivity of Streptococcus mutants to nanoparticles of silver, zinc oxide, and gold.J.Nanomed-Nanotechnol 2008; 49(3): 237-240.
- Nadagouda MN, Hoag G, Collins J, Varma RS. Green synthesis of Au nanostructures at room temperature using biodegradable plant surfactants.J.Cryst Growth Des 2009; 9: 4979- 4983.
- KarimiJ, Mohsenzadeh S. Plant synthesis of silver nanoparticles by Achilleawilhelmsii Pharmaceutical plant. Razi J. of Medical Sciences 2013; 20: 65- 69.
- Kalimuthu K, Suresh R, Venkataraman D, Bilal,M, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis.J. Colloids Surf B Bio interfaces 2008; 65: 150-153.
- Feng Q, Wu J, Chen G, Cui F, Kim T, Kim J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed Mater Res 2000; 52: 662-668.
- RahimiGh, Khodavandi A, Jannesar R, Alizadeh F, Yaghobi R, Sadri A. Evaluation of Antifungal Effects of Ethanolic and AqueousExtracts of ZatariamultifloraHerb in the Pathogenic YeastCandidaalbicansBiofilm Inhibition. J. OF PURE AND APPLIED MICROBIOLOGY 2014; 8: 4559-4564.
- David B. How meaningful are the resuls of nanotoxicitystudies in the absence of adequate material characterilaztion.J.Toxicol sciences2008; 101: 183-185.
- Prashant M, Nisha R, Sudesh K. Biosynthesis of nanoparticles: technological concepts and future application. J. Nanoparticle Research 2007; 10: 507-517.
- Klaus T, Joerger R, Olsson E, Granqvist,CG. Lactobacillus assisted synthesis of titanium nanoparticles. J.Proc.Natl.Acad.Sci.USA 1999; 96: 13611-19.
- Mukherjee P, Ahmad A, Mondol D, Senapati S, Sainkar SR, Khan ML, etal. Fungus mediated synthesis of silver nanoparticles and their immobilization in the mycelia matrix: A novel biological approach to nanoparticle synthesis. J. Nano Lett 2001; 1: 515-19.
- Joerger R, Klaus T, Granqvist,CG. Biologically produced silver-carbon composite materials for optically functional thin-filmcoatings.J. Adv Mater 2000; 12: 407-409.
- Ma H, Yin B, Wang S, Jiao Y, Pan W, Huang S, et al. Synthesis of silver and gold nanoparticles by a novel electrochemical method. J.ChemPhysChem 2004; 5: 68-75.
- Souza G.H, Marcato PD, Duran N, Esposito E. Utilization of Fusariumoxysporum in the biosynthesis of silver nanoparticles and its antibacterial activities. Presentedat Xth National Meeting of Environmental Microbiology.J. Curtiba, PR Brazil. 2004; p 25.
- Shankar SS, Absar A, Murali S. Geranium leaf assisted biosynthesis of silver nanoparticles. J.Biotechnol. Prog 2003; 19: 1627-1631.
- Kathiresan K, Manivannan S, Nabeel MA, Dhivya B. Studies on Silver Nanoparticles Synthesized by A marine fungus, PenicilliumFellutanum Isolated from Coastal Mangrove Sediment. J.Colloids Surf B 2009; 71: 133-134.
- Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts.J.BiopressBiosyst Eng2009; 32: 79-84.