Research Article - Journal of Clinical and Experimental Toxicology (2018) Volume 2, Issue 1
Placental toxicity in rats after administration of broncholytic drug (Mucophylline). A morphological, histological, histochemical and immunohistochemical studies.
Heba Ali1, Abd El Wahab El Ghareeb1, EL-Sayed Fahim Taha1, Hamida Hamdi1,2*
1Department of Zoology, Faculty of Science, Cairo University, Egypt
2Biology department, Faculty of Science, Taif University, Saudi Arabia
- *Corresponding Author:
- Hamida Hamdi
Department of Zoology
Cairo University, Egypt
E-mail: Hamida@sci.cu.edu.eg
Accepted date: February 18, 2018
Citation: Ali H, Ghareeb AEWE, Taha ELSF, et al. Placental toxicity in rats after administration of broncholytic drug (Mucophylline) A morphological, histological, histochemical and immunohistochemical studies. J Clin Exp Tox. 2018;2(1):1-9.
Abstract
Mycophylline is a mucolytic and broncholytic drug can be used in women during pregnancy for the treatment of bronchospasm symptoms, the potential effects induced by this drug in the placenta are not reported. Our study has been designed to evaluate the safety of Mucophylline in different doses (Therapeutic dose), on the rat placenta. The pregnant rats orally administered from 5th until 19th day of gestation with doses 30.83 mg/Kg and 66.61 mg/Kg of Mycophylline (human equivalent dose) daily. On the twentieth day of gestation, the pregnant rats were sacrificed and the placental weights of different groups were recorded. Histological, histochemical, immunohistochemical investigations of placenta, were assessed. Additionally, the oxidative stress markers were assessed. Our results revealed that mucophylline caused the reduction in placental weight was statistically significant in the pregnant rats treated with high dose of mucophylline. histopathologically mucophylline caused an increase number of giant cells, Pycnotic cells, Cystic degeneration in glycogen cells, deposition of fibrin and degeneration of the fetal blood vessels in the labyrinth zone. histochemically, both treated groups showed decrease in amount of carbohydrates in basal and labyrinth layers of the placental tissue. Immunohistochemically, mucophylline caused caspase-3 expression (this is an apoptotic marker) in the placenta. Moreover, Mucophylline elevated malondialdehyde content, reduced the activity of the antioxidant enzymes glutathione reductase, glutathione peroxidase, catalase and the level of non-enzymatic antioxidant reduced glutathione. Our results concluded that Mucophylline medication during pregnancy could possibly cause placental toxicity. Finally, the present work recommended that the pregnant women must avoid the up taking of this bronchodilator drug.
Keywords
Pregnant albino rats, placenta, apoptosis, caspase-3, oxidative stress
Introduction
Throughout the world, asthma affects 2% to 13% of pregnancies [1,2]. Asthma is the most common respiratory condition of pregnancy, with increasing health-care utilization and cost [3]. Placental abruption and placenta previa can be caused by Asthma [4,5].
Mucophylline syrup (Bromhexine hydrochloride 4 mg/5 ml and Acephylline piperazine 100 mg/5 ml), it used in Cough especially when attendant by bronchospasm in such conditions as: asthma, emphysema, acute & chronic bronchitis, chronic inflammatory lung diseases & inhalation of irritants, etc.
During the pregnancy, the placenta acts as a respiratory organ, digestive tract and excretory organ for the fetus; it also provides endocrine and immunity functions that are necessary for the maintenance of a successful pregnancy. It is a temporary and fateful organ for proper fetal development. It is the link between the mother and her fetus [6-8].
It is known that nearly all drugs cross the placenta at least to some extent [9] currently there is little information concerning the pharmacokinetics of Mycophylline in the feto-placental unit, and the changes induced by the drug in the placenta are not reported. The aim of our study is to evaluate the safety of Mucophylline (at therapeutic dose) on rat, Rattus norvegicus placenta by histological, histochemical, immunohistochemical investigations.
Materials and Methods
Experimental design
Thirty healthy adult female albino Wistar rat, Rattus norvegicus (about 11-13 weeks old) were chosen for the current study. The rat has short duration of pregnancy (about 21 days), high fertility rate and large number of litters, genetic stability and a very low rate (about 0.1%) of spontaneous malformation, so it is preferred in teratological studies [10-13]. The standard guidelines of The Institutional Animal Care and Use Committee (IACUC) were implemented in handling the animals. Every morning, vaginal smears were prepared and investigated under the light microscope according to the method of [14] for 5 days to select the female with regular estrus. Two females were selected and caged together with one male overnight under manipulated environmental condition of temperature (25 ± 2ºC), humidity (60 ± 20%) and light (12 light-12 dark cycles). The first day of gestation was confirmed by the presence of sperms in the vaginal smear [15]. Following perception of a sperm plug, pregnant rats were caged individually. Mucophylline was purchased from Misr Co. For Pharm. Ind. S.A.E. – Egypt. Drug orally administrated daily from 5th to 19th day of gestation. The pregnant rats were randomly divided into three groups with 10 rats in each. Group (A) (Control): pregnant rats received distilled water orally. Group (B) (treated): Pregnant rats orally administered with 30.83 mg/kg (low Therapeutic dose) of body weight of mucophylline. Group (C) (treated): Pregnant rats orally administered with 61.66 mg/kg (Therapeutic dose ) of body weight of mucophylline. On the 20th day of gestation, pregnant rats of all groups (A-C) were sacrified by decapitation. All placentas were weighted.
Histological studies
The placenta of different groups (5 tissues /group) were fixed in 10% formol saline for at least one day and then preserved in 70% ethyl alcohol. Placental tissues were dehydrated in ascending series of alcohol, cleared in terpineol and embedded in paraffin wax. Transverse sections 5 μ thick of placental tissues were cut, mounted and stained with haematoxylin and eosin.
Histochemical studies
The prepared sections of placenta were de-paraffinized and then hydrated. These sections were oxidized in 1% aqueous periodic acid for 5 min, then washed in running water for 5 min and rinsed in diluted water. They were put in Schiff's reagent for 10-20 min and then washed in running water for 30 min, and rinsed three times in 0.5% freshly prepared, aqueous sodium metabisulphite and washed in running water for 10 min Nuclei were stained in Celestine blue-haemalum sequence, differentiated in acid alcohol and bleached in tap water. Thereafter, the sections were dehydrated in alcohol, cleared in xylene and mounted in Canada balsam.
Immunohistochemisrtical studies for activated caspase-3
De-paraffinize and hydrate sections of placenta. Theses sections were used for capase-3 (apoptotic marker) according to streptavidinbiotin- peroxidase staining method [16]. Monoclonal caspase-3 (Biovision-3015-100, dilution: 1/25) primary antibodies and biotinylated secondary antibody (DAKO-Universal LSAB Kit-K0690) were used. Caspase-3 positive reactions were visualized and assessed by high-power bright field light microscopic of approximately X20 objective.
Oxidative stress investigation
The placental samples of different groups were stored at -40ºC for oxidative stress investigation. At the moment of investigation, placental samples were thawed, weighted and homogenized in 10 mmol/L phosphate buffer saline (PBS) as 10% (W/V) at pH 7.4. The homogenates were centrifuged and the supernatants were taken for the estimation of (Table 1):
| 1 | Glutathione Reduced (GSH) | 4 | Glutathione Reductase (GR) |
| 2 | GlutathionE-S-Transferase(GST) | 5 | Catalase (CAT) |
| 3 | Glutathione Peroxidase (GX) | 6 | Lipid Peroxidation (MAD) |
Table 1. The homogenates were centrifuged and the supernatants were taken for the estimation.
Estimation of glutathione reduced (GSH)
Tissue GSH was determined by calorimetric method using reagent kits obtained from Bio Diagnostic (Egypt) by the method of Beutler et al. [17].
Estimation of glutathione-s-transferase
Tissue Glutathione-S-Transferase was determined by UV method using reagent kits obtained from Bio Diagnostic (Egypt) by the method of Habig et al. [18].
Estimation of glutathione peroxidase
Tissue Glutathione peroxidase was determined by UV method using reagent kits obtained from Bio Diagnostic (Egypt) by the method of Paglia et al. [19].
Estimation of glutathione reductase
Tissue Glutathione reductase was determined by UV method using reagent kits obtained from Bio Diagnostic (Egypt) by the method of Golgberg et al. [20].
Estimation of catalase
Tissue catalase was determined by calorimetric method using reagent kits obtained from Bio Diagnostic (Egypt) by the method of Aebi et al. [21].
Estimation of lipid peroxidation (Malondialdehyde)
Principle: Thiobarbituric acid (TBA) reacts with malondialdehyde (MDA) in medium at temperature of 95ºC for 30 min to form thiobarbituric acid reactive product the absorbance of the resultant pink product can be measured at 534 nm by the method of Satoh K and Ohkawa et al. [22,23].
Statistical analysis
Data were expressed as mean (μ) ± standard errors of the means (SEM). Statistical analysis was done with the help of the SPSS version 16 (Chicago, IL, USA), while the graphs were drawn using a prism computer program (Graph Pad software Inc V5, San Diego, CA, USA). Data were analyzed statistically by the t-test followed by unpaired Test. Results were considered as statistically significant at (a =P ≤ 0.05).
Results
Placental weight
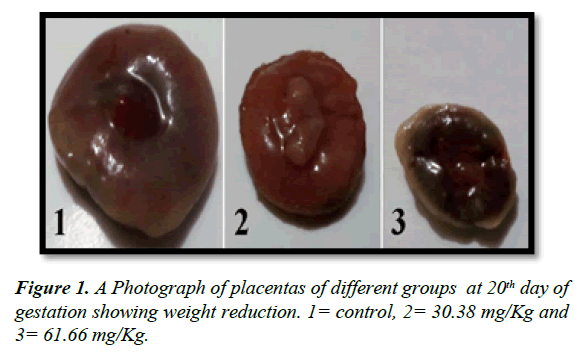
The placental average weight of all groups was decreased as compared to control as in Table 2 and Figure 1. There is a significant decrease (P ≤ 0.05) in placental weight of pregnant rats that received high dose of mucophylline (group C).
| Group | Placenta weight (P.WT) |
|---|---|
| Control (A) | 0.56± 0.007 |
| 30.83 mg/Kg (B) | 0.53± 0.016 |
| 61.66 mg/Kg (C) | 0.52± 0.01a |
Table 2. Showing effect of mucophylline on placental weight at 20th day of gestation.
Histological observations
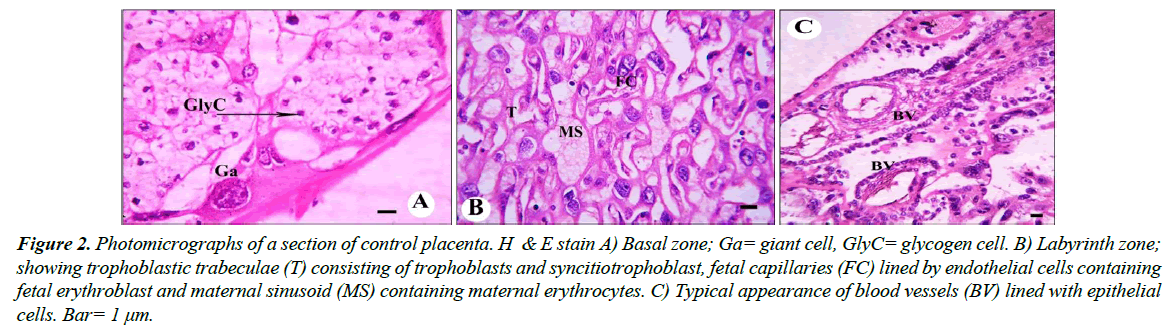
Control group: Histologically, the normal placenta of rats is constructed of the labyrinth zone, the basal zone, the decidua and the metrial glands. The basal zone is consisted of three types of differentiated cells: (1) spongiotrophoblasts, (2) trophoblastic giant cells and (3) glycogen cells (Figure 2A).
The spongiotrophoblasts are present immediately above the trophoblastic giant cell layer located at the materno-fetal placental interface. The glycogen cells form multiple small cell masses and develop into glycogen cell islands. In the labyrinth zone, there are three layers of trophoblasts, separating the maternal blood spaces from the fetal blood vessels (Figure 2B). Also blood vessels appeared in its normal structure (Figure 2C). The outer trophectoderm, which comes into direct contact with the maternal blood, is referred to as cytotrophoblasts with a microvillous surface. Under this trophectoderm, there are two layers of syncytiotrophoblasts. The decidua is composed of the mesometrial decidual cells.
Figure 2: Photomicrographs of a section of control placenta. H & E stain A) Basal zone; Ga= giant cell, GlyC= glycogen cell. B) Labyrinth zone; showing trophoblastic trabeculae (T) consisting of trophoblasts and syncitiotrophoblast, fetal capillaries (FC) lined by endothelial cells containing fetal erythroblast and maternal sinusoid (MS) containing maternal erythrocytes. C) Typical appearance of blood vessels (BV) lined with epithelial cells. Bar= 1 μm.
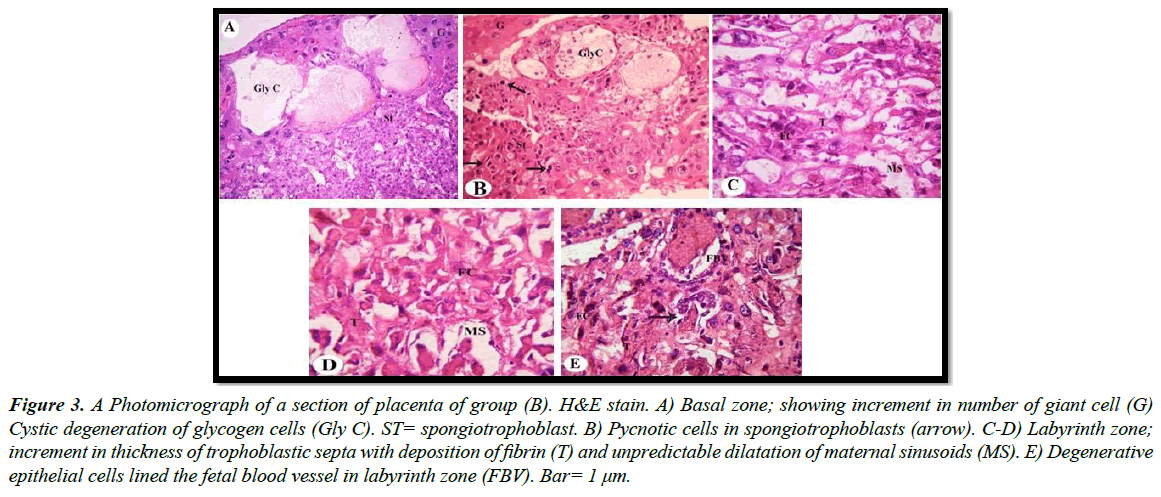
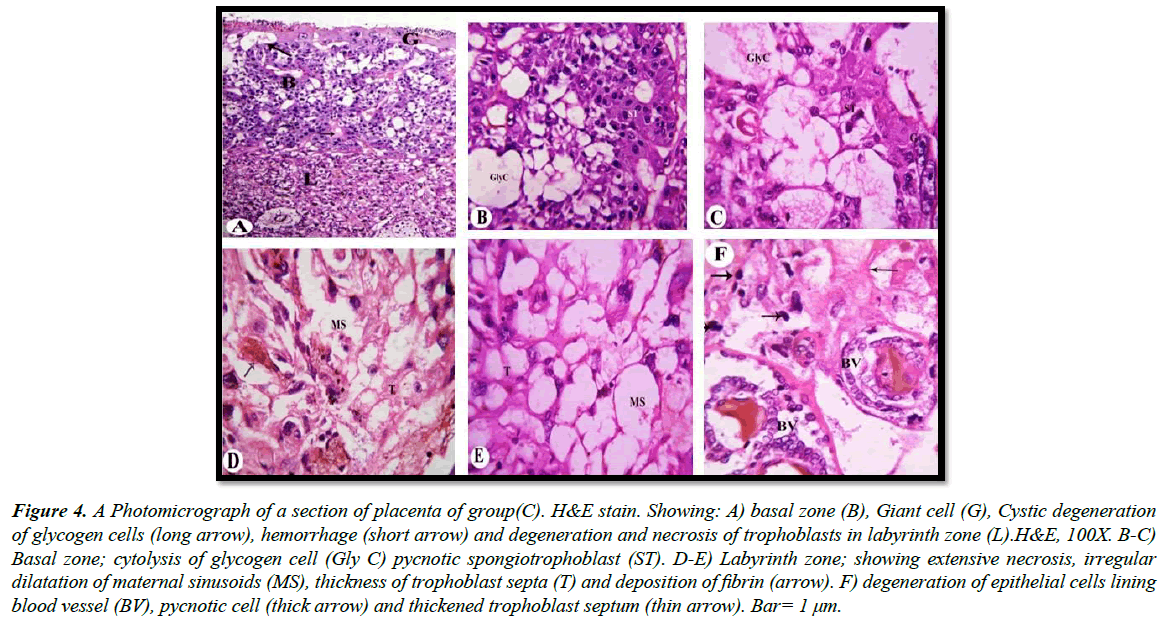
a) Treated groups: Our light microscopic examination showed that increased number of giant cells, Pycnotic cells in the placenta of rats treated with 30.83 mg/Kg, and a marked decrease in glycogen cell-islands in the basal zone. Glycogen cells have Cystic degeneration. Where abnormal retention of extened cytoplasmic vacuolation within glycogen cells. These vacuoles contain eosinophilic fibrinous material and polymorphs. The degenerated cells undergo cytolysis and subsequently coalesce into multiple large cysts that are filled with a homogeneous acidophilic mass and multiple clusters of residual glycogen cells, macrophages, erythrocytes and cell debris (Figure 3A and 3B). Apoptotic cells, characterized by pyknosis or karyorrhexis, phagocytosis and cell debris resulting in labyrinth zone hypoplasia. In addition, irregular dilatation of maternal sinusoids with hemorrhage was observed (Figure 3C and 3D). Degenerative epithelial cells lined the fetal blood vessel in the labyrinth zone (Figure 3E). Light microscopic examination revealed that numerous apoptotic spongiotrophoblast cells were scattered, hemorrhagic areas in between the spongiotrophoblast cells of the basal zone (Figure 4A) in the placenta of rats treated with 61.66 mg/Kg. Cystic degeneration in Glycogen cells (Figure 4B and 4C).
Figure 3: A Photomicrograph of a section of placenta of group (B). H&E stain. A) Basal zone; showing increment in number of giant cell (G) Cystic degeneration of glycogen cells (Gly C). ST= spongiotrophoblast. B) Pycnotic cells in spongiotrophoblasts (arrow). C-D) Labyrinth zone; increment in thickness of trophoblastic septa with deposition of fibrin (T) and unpredictable dilatation of maternal sinusoids (MS). E) Degenerative epithelial cells lined the fetal blood vessel in labyrinth zone (FBV). Bar= 1 μm.
In the labyrinth zone, degeneration and necrosis of the trophoblasts, a decrease in thickness of the trophoblastic septa with a deposition of calcium and irregular dilation of the maternal blood space containing some thrombi, deposition of fibrin (Figure 4D and 4E). Degeneration of the fetal blood vessels in the labyrinth zone (Figure 4F).
Figure 4: A Photomicrograph of a section of placenta of group(C). H&E stain. Showing: A) basal zone (B), Giant cell (G), Cystic degeneration of glycogen cells (long arrow), hemorrhage (short arrow) and degeneration and necrosis of trophoblasts in labyrinth zone (L).H&E, 100X. B-C) Basal zone; cytolysis of glycogen cell (Gly C) pycnotic spongiotrophoblast (ST). D-E) Labyrinth zone; showing extensive necrosis, irregular dilatation of maternal sinusoids (MS), thickness of trophoblast septa (T) and deposition of fibrin (arrow). F) degeneration of epithelial cells lining blood vessel (BV), pycnotic cell (thick arrow) and thickened trophoblast septum (thin arrow). Bar= 1 μm.
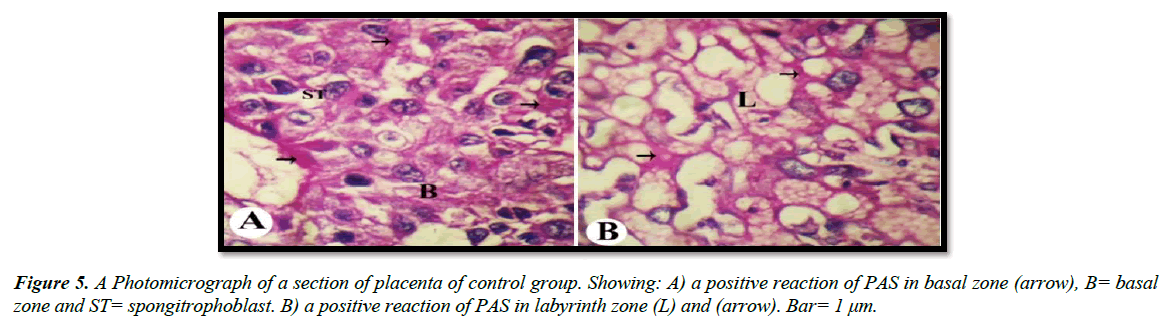
Histochemical observations (PAS)
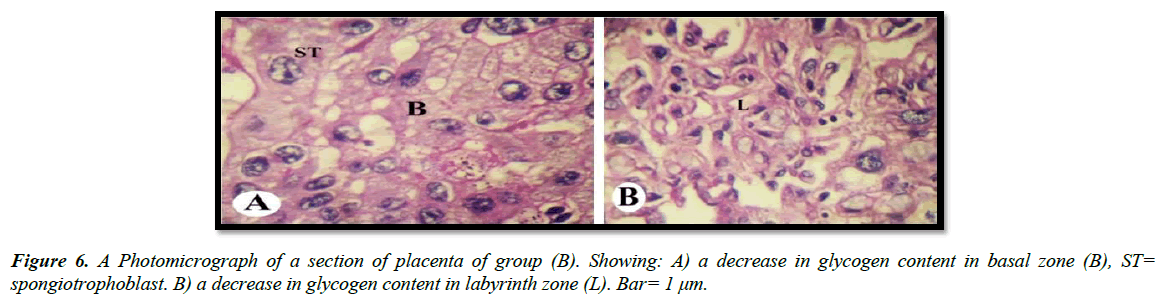
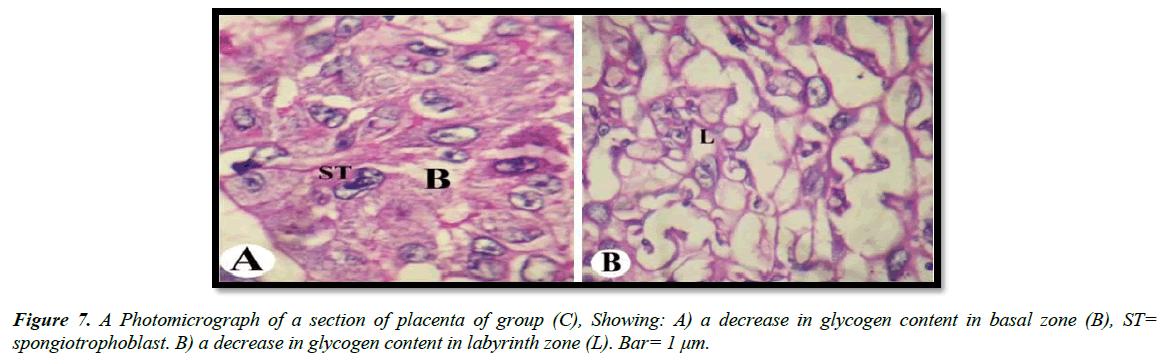
The light microscopic examinations revealed that, the cytoplasm of spongitrophoblast cells of the basal zone, trophoblastic septa and syncytiotrophoblast of the labyrinth zone of the placental tissue of the control group had positive PAS reaction (Figure 5A and 5B). The placental tissue sections of pregnant rats treated with 30.83 mg/Kg of mucophylline group (B) revealed a noticed depletion of the glycogen content in the basal and labyrinth layers (Figure 6A and 6B). The basal zone of the placenta group (C) exhibited a less content of carbohydrates but not such as the placental tissue of group (B), while the labyrinth zone of the treated group (c) showed a marked decreased in glycogen content than that from group (B )(Figure 7A and 7B).
Immunohistochemisrty observations
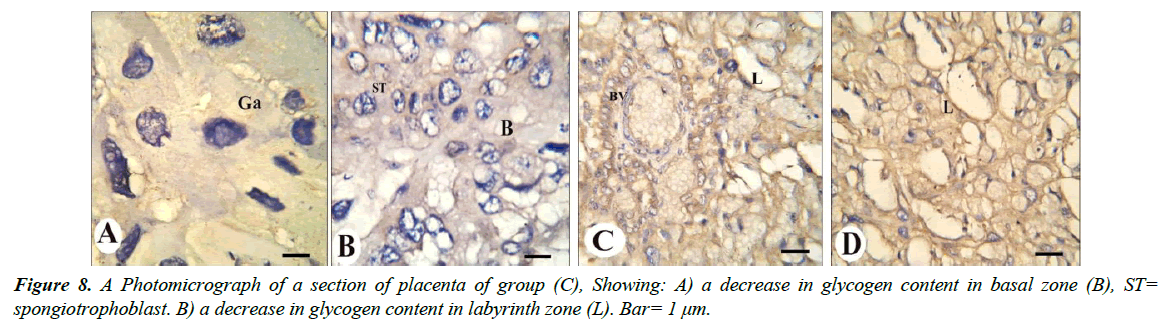
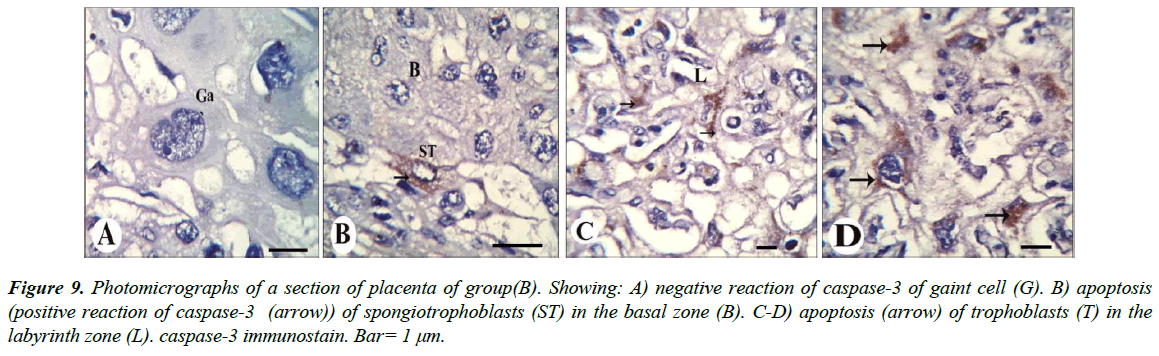
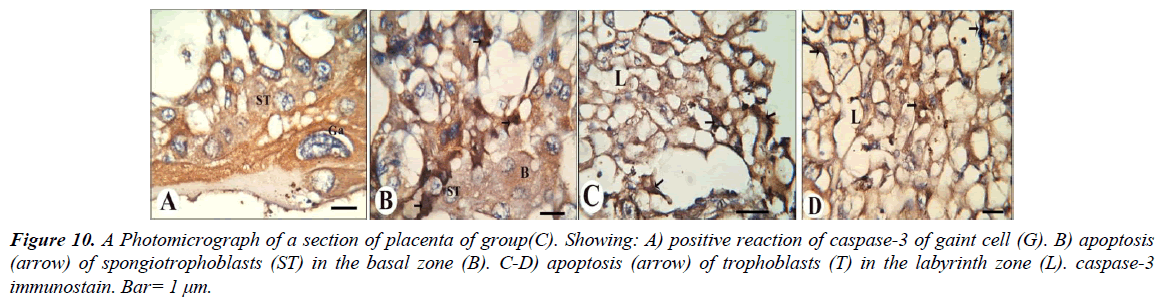
Our results revealed that, gaint cells and spongitrophoblast cells of the basal zone of placenta of the control group showed negative reaction of caspase-3 (Figure 8A and 8B), also trophoblastic septa and syncytiotrophoblast of the labyrinth zone showed negative reaction (Figure 8C and 8D). The gaint cells of placental sections of rats treated with 30.83 mg/Kg of mucophylline showed negative reaction of caspase-3 (Figure 9A), spongitrophoblast cells of basal zone showed positive caspase-3 reaction (Figure 9B) and trophoblastic septa and cytoplasm of syncytiotrophoblast cells of the labyrinth zone, showed positively caspase-3 activity in (Figure 9C and 9D). cytoplasm of the giant cells of The basal zone of the placental sections of rats treated with 61.66 mg/Kg showed a highly positively caspase-3 activity (Figure 10A), spongitrophoblast cells (Figure 10B) and in the trophoblastic septa of labyrinth zone of the same group, showed a highly apoptotic index (Figure 10C and 10D).
Figure 9: Photomicrographs of a section of placenta of group(B). Showing: A) negative reaction of caspase-3 of gaint cell (G). B) apoptosis (positive reaction of caspase-3 (arrow)) of spongiotrophoblasts (ST) in the basal zone (B). C-D) apoptosis (arrow) of trophoblasts (T) in the labyrinth zone (L). caspase-3 immunostain. Bar= 1 μm.
Figure 10: A Photomicrograph of a section of placenta of group(C). Showing: A) positive reaction of caspase-3 of gaint cell (G). B) apoptosis (arrow) of spongiotrophoblasts (ST) in the basal zone (B). C-D) apoptosis (arrow) of trophoblasts (T) in the labyrinth zone (L). caspase-3 immunostain. Bar= 1 μm.
Oxidative stress observations
The data in Table 3 revealed that, pregnant rats treated with 30.83 mg/Kg of mucophylline showed no significant decrease in GSH level (P ≥ 0.05). However, pregnant rats treated with 61.66 mg/ Kg of mucophylline showed a significant decrease (P ≤ 0.05) in the level of GSH when compared with the control group. Oral administration of low and high doses of mucophylline revealed no significant decrease in the levels of antioxidant enzymes. Pregnant rats exposed to 30.83 mg/Kg of mucophylline showed significant decrease in the catalase activity when compared with control group, while there was no significant decrease in catalase level of pregnant rats treated with high dose (61.66 mg/ Kg). The level of MDA was elevated as an indicator of lipid peroxidation. In both treated groups a significant increase (P ≤ 0.05) in the levels of MDA were observed when compared to the control group. There was no significant difference in all parameters of oxidative stress between two treated groups.
| Group | GA (control) | GB (30.83 mg/Kg) | GC (61.66 mg/Kg) |
|---|---|---|---|
| Parameter | |||
| GSH (mg/g) | 11.09± 0.14 | 8.1343± 2.08 | 7.89± 0.62a |
| GST (U/g) | 2.32± 0.02 | 2.051± 0.34 | 1.98±0.60 |
| GX (U/g) | 1536.81±269.55 | 870.53± 64.57 | 628.98± 172.20 |
| GR (U/L) | 397.87±164.69 | 215.34± 10.00 | 217.01± 12.18 |
| CAT (U/g) | 2.25± 0.07 | 1.56± 0.17a | 2.15± 0.00 |
| MDA (nmol/g) | 114.64± 7.50 | 166.96± 6.28a | 171.36± 9.87a |
Table 3. Showing effect of mucophylline on placental antioxidant system and MDA of pregnant rats at 20th day of gestation. Values are expressed as Mean ± SEM. The statistical differences were analyzed by ANOVA followed by independent samples T test. a= P ≤ 0.05 compared with control.
Discussion
Biological significance in the pregnant maintenance and prenatal life can be affected by Placental development and function. Hence, chemical-induced alteration or deviation of placental function in the maternal and extraembryonic tissue can ultimately lead to pregnancy loss, congenital anomalies and fetal death. Drug- or chemical- induced histopathological changes of the placenta in rats are essential in safety evaluation to understand the mechanism of teratogenicity and developmental toxicity. However, the placenta has not received proper consideration as a target organ in safety assessment for dams and embryos/fetuses. Morphological or histopathological assessment of placental development and abnormalities has been rare and incomplete in experimental animals [24,25].
Placental weight
The current study revealed that the reduction in placental weight was statistically significant in the pregnant rats treated with high dose of mucophylline. As has been reported previously that small placenta leads to the reducation in uterine blood flow to the placenta and transportation to the fetuses, which is the main cause of fetal growth retardation [26].
Histological studies
The current study showed the increased number of giant cell in the spongy zone is found to be a regular feature of all experimental placentas. The giant cells have several functions such as phagocytosis and steroid hormone production. The occurrence of phagocytosis in the spongy zone, in the areas where glycogen cells were degenerating resulting in cyst formation as has been reported previously in Padmanabhan et al. [27]. The trophoblast cells of the labyrinths also produced a fibrinoid substance, which appeared to be secreted into the maternal sinusoid. Fibrin could also have developed as a result of enhanced vascular leakage due to mucophylline administration. fibrinoid accumulation in the labyrinths and the altered labyrinthine architecture, poor vascularization, would suggest a substantial reduction in placental transport functional capacity and, consequently, lead to fetal growth retardation.
Abnormalities in placental structure and the consequent function changes could secondarily affect fetal growth, as has been reported previously in some experimental and clinical studies [28-34].
Histochemical observations (PAS)
In our studies, both treated groups revealed that decrease in amount of carbohydrates in basal and labyrinth layers of the placental tissue as a result of glycogen cells degeneration as has been reported previously Padmanabhan et al. [27].
Immunohistochemical studies
Apoptosis is a programmed cell death in which a cell actively induces its own death. But an imbalance of this process in placenta leads to placental dysfunction [35]. Placental dysfunction causes the insufficient supply of nutrients and oxygen to support normal growth of the fetus, resulting in fetal blood flow redistribution [36,37]. Thus, it is feasible that apoptosis in placenta may be related to Congential Talipes Equinovarus [38]. Our results revealed that a caspase-3 activity in the placental tissue, especially in the labyrinth zone, of the two treated groups compared to the control group. Apoptosis is characterized by DNA fragmentation, membrane blebbing, cell shrinkage, and disassembly into membrane-enclosed vesicles, which prevents a host inflammatory response to intracellular components [39]. At molecular level, classical apoptosis is caused by the activation of a family of cysteine protease known as caspases that cleave their target protein at specific aspartic acids. Two major apoptotic pathways culminate in the activation of downstream executioner caspases-3 and 6 [40,41]. So caspase-3 is an immunohistochemistry marker to apoptosis [42]. Our studies in concurrence with Erboga et al. [43] concluded that cadmium administered during the pregnancy caused abnormal placental development by disrupting the normal structure of the placenta, inhibiting the proliferation of trophoblast and increasing the number of apoptotic trophoblast cells. In addition, the trophoblasts in the labyrinth zone, at which the maternal and fetal bloods come very close together, play pivotal roles in the maternal–fetal exchange, placental xenobiotic metabolism, placental endocrine function and placental barrier of substances [44,45]. The labyrinth zone dysfunction is known to cause unfavorable impacts on embryonic development. Subsequently, we speculated that mucophylline actuated growth arrest and apoptosis in the trophoblasts of the labyrinth zone, followed by the placenta dysfunction. Since embryonic/fetal weight gain restriction was observed obviously following withdrawal during the fetal period, as in our previous studies El Ghareeb et al. [46] showed that the mucophylline actuated fetal intra-uterine growth retardation might not only result in anti-proliferative effects of mucophylline during the organogenesis, but also disrupt placental functions during the fetal period. Moreover, the point-of-no-return in the apoptotic signaling cascade is activation of caspase-3 [47]. In this relation, our data revealed a significant increase in activities of caspase-3 in pregnant rats administrated muophylline, reaffirmed their role in apoptotic pathway [48].
Oxidative stress observations
Glutathione-S-transferase (GST) activity is responsible for the conjugation and detoxification of intermediates produced by oxidative stress in rat placenta and is associated with the cell cycle [49]. Mucophylline treated pregnant rats showed a significant decrease in GST activity on 20th day when compared to the corresponding control group. The placental tissue showed significant decrease in CAT at the low dose and no significant depletion in GR and GX at both treated groups. The induction of oxidative stress in the fetal system is the essential mechanism of induction of fetal toxicity by teratogenic agents [50]. Oxidative stress can cause cell death and even moderate oxidation can trigger apoptosis, while more intense stresses may cause necrosis [51,52]. It is known that the oxidative stress occurs as a consequence of imbalance between the production of ROS and antioxidative process in favor of free radical production [53]. Moreover, inhibition of enzymes involved in removal of free radicals might result in accumulation of H2O2, which has been suggested to promote lipid peroxidation, modulation of DNA, altered gene expression and cell death [54,55].
Our results revealed that mucophylline caused the reduction in placental weight was statistically significant in the pregnant rats treated with high dose of mucophylline. Histopathologically, mucophylline caused an increase number of giant cells, Pycnotic cells, Cystic degeneration in glycogen cells, deposition of fibrin and degeneration of the fetal blood vessels in the labyrinth zone. histochemically, both treated groups showed decrease in amount of carbohydrates in basal and labyrinth layers of the placental tissue. Immunohistochemically, mucophylline caused caspase-3 expression (this is an apoptotic marker) in the placenta. Moreover, Mucophylline elevated malondialdehyde content, reduced the activity of the antioxidant enzymes glutathione reductase, glutathione peroxidase, catalase and the level of non-enzymatic antioxidant reduced glutathione. Our results concluded that Mucophylline medication during pregnancy could possibly cause placental toxicity. Finally, the present work recommended that the pregnant women must avoid the up taking of this bronchodilator drug.
References
- Jolving LR, Nielsen J, Kesmodel US, et al. Prevalence of maternal chronic diseases during pregnancy- a nationwide population based study from 1989 to 2013. ActaObstetGynecolScand 2016;95:1295-1304.
- Sawicki E, Stewart K, Wong S, et al. Medication use for chronic health conditions by pregnant women attending an Australian maternity hospital. Aust N Z J ObstetGynaecol 2011;51:333-8.
- Kim S, Kim J, Park SY, et al. Effect of pregnancy in asthma on health care use and perinatal outcomes. J Allergy ClinImmunol2015;136:1215-23.
- Mendola P, Laughon SK, Mannisto TI, et al. Obstetric complications among US women with asthma. Am J ObstetGynecol2013;208:127.e1-127.e8.
- Wang G, Murphy VE, Namazy J, et al. The risk of maternal and placental complications in pregnant women with asthma: a systematic review and meta-analysis. J MaternFetal Neonatal Med 2014;27:934-42.
- Boyd CA. Review: Epithelial aspects of human placental trophoblast. Placenta2013;34:S24-6.
- Carter AM. Evolution of placental function in mammals: the molecular basis of gas and nutrient transfer, hormone secretion, and immune responses. Physiol Rev 2012;92:1543-76.
- Malek A. Role of IgG antibodies in association with placental function and immunologic diseases in human pregnancy. Expert Rev ClinImmunol2013;9:235-49.
- Myllynen P, Pienimäki P, Vähäkangas K. Human placental perfusion method in the assessment of transplacental passage of antiepileptic drugs. ToxicolApplPharmacol 2005; 207:489-94.
- Banerjee BN, Durloo RS. Incidence of teratological anomalies controls charles River C-D strain rats. Toxicology1973; 1:151-4.
- Tuchmann-Duplessis H. Methods for evaluating teratogenic properties of new drugs. In: "Methods in Drug Evaluation" North-Holand publishing company 1966.
- Tuchmann-Duplessis H. Drug effect on the fetus: General principles of drug induced congenital abnormalities. ADIS Press, New York1977; p:39.
- Wilson JG. Environment and birth defects. Academic press, New York 1973.
- Snell GD. Biology of the laboratory Mouse, 5thed. The Blakiston Company: Philadelphia 1956.
- McClain RM, Becker BA. Teratogenicity, foetal toxicity and placental transfer of lead nitrate in rats. ToxicolApplPharmacol 1975; 931:72-82.
- Karakus E, Karadeniz A, Simsek N, et al. Protective effect of Panax ginseng against serum biochemical changes and apoptosis in liver of rats treated with carbon tetrachloride (CCl4). J Hazardous Materials2011;195:208-13.
- Beutler E, Duron O, Kelly MB. J Lab Clin Med 1963;61:882.
- Habig W, Pabst M, Jakoby W. Glutathione S-TransferasesThe First Enzymatic Step In Mercapturic Acid Formation. J BiolChem 1974; 249:7130-9.
- Paglia DE, Valentine W N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med1967;70:158-69.
- Golgberg DM, Spooner RJ. In Methods of Enzymatic Analysis (Bergmeyen, H.V. Ed.) 3rdedn.VerlogChemie Deerfield beach Fl1983; 3:258-65.
- Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121-6.
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. ClinicaChimicaActa 1978; 90:37-43.
- Ohkawa H, Ohhhishi W, Yagi K. Anal Biochem 1979;95:35.
- Furukawa S, Hayashi S, Usuda K, et al. Toxicological Pathology in the Rat Placenta. J ToxicolPathol2011;24:95-111.
- Hala ME. Histopathological effects of experimental phenylketonuria on 15 days albino rat placenta. Life Science Journal2013;10:1759-66.
- Garnica AD, Chan WY. The role of the placenta in fetal nutrition and growth. J Am CollNutr1996; 15:206-22.
- Padmanabhan R, AL-MenhaliM, Ahmed I , et al. Histological histochemical and electron microscopic changes of the placenta induced by maternal exposure to hyperthermia in the rat. International Journal of Hyperthermia 2005;21:29-44.
- Padmanabhan R, Shafiullah M. Intrauterine growth retardation in experimental diabetes: Possible role of the placenta. Arch PhysiolBiochem 2001;109:260-71.
- Padmanabhan R. Histological and histochemical changes of placenta in fetal alcohol syndrome due to maternal administration of acute doses of ethanol in the mouse. Drug Alcohol Depend 1985;16:229-39.
- Wigglesworth JS. Fetal growth retardation. Animal model: Uterine vessel ligation in the pregnant rat. Am J Pathol 1974;77:347-50.
- Sreenathan RN, Singh S, Padmanabhan R. Implication of the placenta in acetaldehyde-induced intrauterine growth retardation. Drug Alcohol Depend 1984;13:199-204.
- Padmanabhan R. The effect of cadmium on placental structure and its relation to fetal malformations in the mouse. Z MikroskAnatForsch 1986; 100:419-27.
- Ashworth CJ, Finch AM, Page KR, et al. Causes and consequences of fetal growth retardation in pigs. ReprodSuppl 2001;58:233-46.
- Chen CP, Bajoria R, Aplin JD. Decreased vascularization and cell proliferation in placentas of intrauterine growth-restricted fetuses with abnormal umbilical artery flow velocity waveforms. Am J ObstetGynecol 2002;187:764-9.
- Daher S, Guimaraes AJ, Mattar R, et al. Bcl-2 and Bax expressions in pre-term, term and post-term placentas. Am J ReprodImmunol2008; 60:172-8.
- Sundberg K, Bang J, Smidt-Jensen S, et al. Randomised study of risk of fetal loss related to early amniocentesis versus chorionic villus sampling. Lancet 1997;350:697-703.
- Peebles DM. Fetal consequences of chronic substrate deprivation. SeminFetal Neonatal Med 2004; 9:379-86.
- Bo J, Zhiqun Z, Pengfei Z, et al. Apoptotic genes expression in placenta of clubfoot-like fetus pregnant rats. Int J ClinExpPathol 2014; 7:677-84.
- Crysns V, Yuan J, Lockshin R, et al. The cutting edge: caspases in apoptosis and disease in When Cells Die, New York1998; pp:177-210.
- Kang JJ, Schaber MD, SrinivasulaSM. Cascades of mammalian caspase activation in the yeast Saccharomyces cerevisiae. Journal of Biological Chemistry 1999;274:3189-98.
- Brito GAC, Fujji J, Carneiro-Filho BA, et al. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. Journal of Infectious Diseases2002; 186:1438-47.
- Jos´e ERHJ, Germana SV, Francisca TSR, et al. Monocrotaline: Histological Damage and Oxidant Activity in Brain Areas of Mice. Oxidative Medicine and Cellular Longevity 2012; pp:1-10.
- Erboga M, Kanter M. Effect of Cadmium on Trophoblast Cell Proliferation and Apoptosis in Different Gestation Periods of Rat Placenta. Biol Trace Elem Res 2016;169:p285.
- Takata K, Fujikura K, Shin B. Ultrastructure of the rodent placental labyrinth: a site of barrier and transport. J Repro Dev1997; 43:13-24.
- Goodman DR, James RC, Harbison RD. Placental toxicology. Food ChemToxicol1982;20:123-8.
- El Ghareeb AE, Hamdi H, Taha EF, et al. Evaluation of Teratogenic potentials of Bronchodilator drug on offsprings of Albino rats. International Journal of Scientific & Engineering Research2015; 6:534-42.
- Green DR, Amarante-Mendes GP. The point-of-no-return: mitochondria, caspases and the commitment to cell death. Cell Differ 1998; 24:45-61.
- Quaiser S, Sabry MA, Maqsood AS, et al. Phorate-induced oxidative stress, DNA damage and transcriptional activation of p53 and caspase genes in male Wistar rats. Toxicology and Applied Pharmacology2012;259:54-65.
- Cervello I, Lafuente A, Giralt M, et al. Enhanced glutathione-Stransferase (GST) activity in pregnant rats treated with benzo(a) pyrene. Placenta 1992; 13:273-80.
- Shepard TH. Teratogenicity of therapeutic agents. CurrProblPediatr1979; 10:1-42.
- Lennon SV, Martin SJ, Cotter TG. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif1991; 24:203-14.
- Awodele O, Popoola TD, Odunsi P, et al. Assessing the Risk of Birth Defects Associated With Exposure to Highly Active Anti-Retroviral Therapy during Organogenesis in Rats. Tokai J ExpClin Med 2013; 38:82-92.
- Dringen R. Metabolism and functions glutathione in brain. ProgNeurobiol 2000;62:649-71.
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine, 3rded. Oxford Sciences Publications1999.
- Fetoui H, Garoui EM, Zeghal N. Lambda-cyhalothrin-induced biochemical and histopathological changes in the liver of rats: ameliorative effect of ascorbic acid. ExpToxicolPathol2009;61:189-96.