Research Article - Biomedical Research (2019) Volume 30, Issue 2
Placental protein 13 and asymmetric dimethyl arginine for early assessment of preeclampsia
Ranjeeta Gadde1, Dayanand CD1* and Sheela SR21Department of Biochemistry, Sri Devaraj Urs Medical College, Sduaher, Tamaka, Kolar, Karnataka, India
2Department of Obstetrics and Gynecology, Sri Devaraj Urs Medical College, Sduaher, Tamaka, Kolar, Karnataka, India
- *Corresponding Author:
- Dayanand CD
Department of Biochemistry
Sri DevarajUrs Medical College
Sduaher, Karnataka, India
Accepted date: February 20, 2019
DOI: 10.35841/biomedicalresearch.30-19-078
Visit for more related articles at Biomedical ResearchAbstract
Objective: To evaluate serum levels of biomarkers as early indicators of onset of preeclampsia during pregnancy. Method: A nested case-control study was conducted among the pregnant women who visited for antenatal check-up at R.L. Jalappa Research Center, Kolar between August 2017 and May 2018. Serum levels of placental protein 13 (PP13), asymmetric dimethylarginine (ADMA), nitric oxide and caspase-3 which represents placentation and endothelial function were measured and compared between trimesters. Results and Discussion: There were 86 pregnant women enrolled at first trimester, the baseline values of women who developed preeclampsia were PP13 (477.54 ± 62.72 pg/ml), ADMA (21.06 ± 19.42 ng/ml), nitric oxide (3.71 ± 2.76 nmol/μL) and caspase-3 (6.15 ± 0.55 ng/ml). In the second trimester on follow up, the mean serum concentration of PP13 was 530.64 ± 69.10 pg/ml in preeclampsia versus 518.26 ± 49.33 pg/ml in normal pregnancy. The mean serum concentration of ADMA was 73.43 ± 30.95 ng/ml and nitric oxide 4.10 ± 2.70 nmol/μL in preeclampsia versus 21.06 ± 19.42 ng/ml and 4.85 ± 2.19 nmol/μL in normal pregnancy. The mean serum concentration of caspase-3 in preeclampsia was 22.65 ± 0.91 ng/ml versus normal pregnancy 18.45 ± 2.62 ng/ml were recorded. In preeclampsia, PP13 was positively correlated with nitric oxide and negatively correlated with ADMA. Conclusion: Decreased PP13, nitric oxide and elevated ADMA in first trimester and increased PP13, ADMA and decreased nitric oxide at second trimester reflects altered placentation and endothelial function.
Keywords
Asymmetric dimethylarginine, Placental protein 13, Preeclampsia, Caspase-3D
Introduction
Preeclampsia contributes to the global incidence of 2-8% of pregnancies with maternal/neonatal morbidity and mortality in developing countries with poor antenatal care. The criteria for diagnosis of preeclampsia are blood pressure ≥ 140/90 mmHg with renal insufficiency, impaired liver function, haematological and neurological complications [1]. The adverse outcomes of preeclampsia are fetal growth restriction with oligohydramnios, preterm birth, low birth weight, severe birth asphyxia, still birth or intrapartum death. Many etiologies linked to this syndrome, viz. insufficient trophoblast invasion, uteroplacentalischemia, vascular disorders of the placenta, insulin resistance, systemic maternal inflammation, endothelial dysfunction and antiangiogenic state [2]. Even though, the exact reason for this pregnancy disorder is not known, preeclampsia research is striving to address this issues related to early diagnosis, underlying mechanism and management of the disorder. Therefore, the early assessment of preeclampsia by using biomarkers is effective and has drawn much attention. Human placental protein 13 (PP13) is 32 kDa β-galactoside binding soluble-type galectin synthesized in the syncytiotrophoblast. The presence of carbohydrate recognition domain (CRD) in its structure enables binding to the glycans of endometrial membrane and annexin II of the extracellular matrix during implantation and embryogenesis. PP13 binds to β and γ actin proteins of cytoskeleton in the syncytiotrophoblast and facilitates its migration by increasing prostaglandin release, which is important for vascular remodelling during placental development. Besides, it is also known to provide immune tolerance at the maternal-fetal interface [3]. Nevertheless, theexact aetiology of preeclampsia is unclear, abnormal remodelling of uterine blood vessels and immunological tolerance between fetal and maternal tissues play possible role in the pathogenesis of this disorder [4]. Therefore, early assessment of PP13 concentrations seems advantageous.
Asymmetric dimethylarginine (ADMA), a methylated compound generated by post translational modification of proteins, that prevents nitric oxide production by competing with L-arginine and inhibits nitric oxide synthase activity. It is associated with endothelial dysfunction in the placenta reducing placental perfusion as seen in preeclampsia. There are reports presenting increased ADMA concentration in first trimester that could predict preeclampsia [5].
Caspases or cysteine aspartic proteases also known as cathepsins are predominantly intracellular enzymes involved in the process of apoptosis. Cellular stress such as hypoxia/ oxidative stress is known to trigger the intrinsic pathway and extrinsic pathway is brought about by the binding of first apoptotic signal-associated death domain (FADD) to its death receptors (Tumor necrosis factor death receptor family) expressed by the trophoblasts. Collectively, both the pathways lead to activation of executioner or effector caspase-3 to initiate apoptosis [6]. Small number of studies has reported the association of serum caspase-3 levels with other diseases like severe traumatic brain injury, intracerebral haemorrhage and endometriosis severity [7-9]. Also a report available on increased expression of caspase-3 in placental bed biopsies have shown to be associated with preeclampsia [10]. The information on serum caspase-3 in pregnancies with or without complications is scarce. Thereby, current study is attempting to evaluate serum caspase-3 in pregnancy.
Nitric oxide, a potent vasorelaxant released by endothelial cells, inhibits platelet aggregation and adhesion to vascular endothelial surfaces. In the syncytiotrophoblast, nitric oxide functions as the main vasodilator of the placenta lowering fetoplacental vascular resistance [11].
Our earlier research findings on screening oxidative stress, biophysical parameter, placental protein 13, ADMA, caspase-3, nitric oxide in first trimester were reported [12,13]. However, as a part of continuation, current study parameters were measured in second trimester to identify women who subsequently developed preeclampsia.
The objective of the study is to screen pregnant women with serum biochemical indicators for understanding the process of placentation and placental vascular endothelial function and also to identify women who possibly develop preeclampsia during their pregnancy.
Materials and Methods
A nested case-control study has enrolment of eighty-six women at 11-24 weeks of pregnancy visited R. L. Jalappa Hospital and Research Centre for antenatal check-up between August 2017 and May 2018. Department of Obstetrics and Gynaecology and Department of Biochemistry participated in the study and the study design was approved by Central ethics Committee of Sri Devaraj Urs Academy of Higher Education and Research, India. Informed consent for participation in the study and blood sampling was obtained from all the participants of the study group. During the study period, subjects were screened for the biochemical parameters in first and second trimesters of pregnancy. Four millilitres of blood was collected from pregnant women by venipuncture under aseptic conditions in first and second trimesters during their regular antenatal check-up. Blood samples were allowed for retraction at room temperature, and centrifuged at 3000 rpm for 20 minutes for separation of clear sera which were stored at -20°C until ELISA was performed. Primigravida aged 20-35 years with singleton pregnancy were included in the study and exclusion criteria was women with history of liver disease, renal failure, hypertension, or with any vascular diseases.
Maternal serum PP13 was measured by ELISA (CUSABIO, USA). This assay employs the competitive inhibition enzyme immunoassay technique. The detection range was 2.5-1000 pg/ml. The minimum detectable dose of human PP13 is typically less than 1 pg/ml. Intra-assay precision was CV %<6% and inter-assay precision: CV%<11%. Maternal serum caspase-3 was also measured by ELISA (CUSABIO, USA). The detection range is 0.312-20 ng/ml. The sensitivity of the test is less than 0.078 ng/ml. The intra-assay precision is CV %<8% and inter-assay precision: CV%<10%. Nitric oxide was determined colorimetrically (Biovision, USA). ADMA was quantified by competitive inhibition enzyme immunoassay technique (ELISA, Sunred Biotechnology Company, Shanghai China,). The detection range is 7.8-500 ng/ml and sensitivity is less than 1.95 ng/ml. The intra-assay precision is CV%<8% and inter-assay precision is CV%<10%.
The obtained data was statistically analysed using licensed version of SPSS 20.0. Mean ± SD was calculated for all the variables. Since the data was not normally distributed, nonparametric Wilcoxon rank sum test was used to find significance between the study parameters in first and second trimesters of pregnancy and cases that developed preeclampsia. Pearson's correlation was used to assess the correlation between PP13, ADMA and Nitric oxide in preeclampsia in both the trimesters. Statistical significance was defined as p<0.05.
Results
The study group enrolled 86 pregnant women; were in the mean age group 24.86 ± 1.33 years. The baseline values of the study parameters were measured during their visit at first and second trimester of ante natal check-up. The parameters measured in first and second trimester were PP13, ADMA, Nitric oxide and caspase 3.
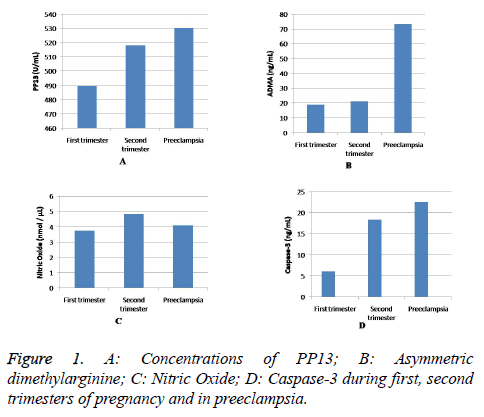
Table 1 shows the comparison of outcome of parameters between first and second trimesters of pregnancy. The mean PP13 levels were 489.77 ± 53.62 pg/ml, ADMA 19.03 ± 17.08 ng/ml, nitric oxide 3.75 ± 2.14 nmol/ml and caspase-3 6.06 ± 0.72 ng/ml in the first trimester. In second trimester, the mean PP13 levels were 518.26 ± 49.33 pg/ml, ADMA 21.06 ± 19.42 ng/ml, nitric oxide 4.85 ± 2.19 nmol/ml and caspase-3 18.45 ± 2.62 ng/ml were observed. As expected, the concentrations of the above parameters increased as gestation progressed and the mean differences were found to be statistically significant (p<0.001).
| Variables | First trimester | Second trimester | p value |
|---|---|---|---|
| Placental protein 13 (pg/ml) | 489.77 ± 53.62 | 518.26 ± 49.33 | <0.001* |
| Nitric oxide (nmol/µL) | 3.75 ± 2.14 | 4.85 ± 2.19 | <0.001* |
| Asymmetric dimethylarginine (ng/ml) | 19.03 ± 17.08 | 21.06 ± 19.42 | <0.001* |
| Caspase-3 (ng/ml) | 6.06 ± 0.72 | 18.45 ± 2.62 | <0.001* |
Table 1. Comparison of outcome of parameters between first and second trimester of pregnancy.
Table 2 shows comparison of outcome of parameters between the baseline values of cases that developed into preeclampsia and also their concentrations in second trimester during their follow-up (n=7). The baseline values of the cases that developed into preeclampsia were almost close to the values of the control group (n=86) in the first trimester except with 10% marginal variation of ADMA.
| Variables | Baseline values of first trimester (n=7) | Cases developed preeclampsia after second trimester (n=7) | p value |
|---|---|---|---|
| Placental protein 13 (pg/ml) | 477.54 ± 62.72 | 530.64 ± 69.10 | <0.05 |
| Nitric oxide (nmol/µL) | 3.71 ± 2.76 | 4.10 ± 2.70 | <0.05 |
| Asymmetric dimethylarginine (ng/ml) | 21.06 ± 19.42 | 73.43 ± 30.95 | <0.05 |
| Caspase-3 (ng/ml) | 6.15 ± 0.55 | 22.65 ± 0.91 | <0.05 |
Table 2. Comparison of outcome of parameters between baseline values of first and cases developed preeclampsia after second trimester of pregnancy.
The baseline mean values and standard deviation of the study parameters in first trimester in women who developed preeclampsia (n=7) were PP13 477.54 ± 62.72 pg/ml, ADMA 21.06 ± 19.42 ng/ml, nitric oxide 3.71 ± 2.76 nmol/ml and caspase-3 6.15 ± 0.55 ng/ml. In second trimester, the mean concentrations of PP13 is 530.64 ± 69.10 pg/ml, ADMA 73.43 ± 30.95 ng/ml, nitric oxide 4.10 ± 2.70 nmol/ml and caspase-3 22.65 ± 0.91 ng/m were observed. Values were higher in the second trimester and the difference in the means was found to be statistically significant (p<0.05). The levels of PP13, caspase-3, ADMA and nitric oxide in first and second trimesters (n=86) and in preeclampsia (n=7) are represented in Figure 1.
Table 3 illustrates the correlation co-efficient (r) and probability value (p) between the study parameters between first and second trimesters. On correlation analysis, a positive significant correlation was observed in first trimester between PP13 and nitric oxide (r=+0.21, p=0.04), however, it was nonsignificant in second trimester (r=+0.19, p=0.06). A nonsignificant negative correlation was also observed between PP13 and ADMA in first (r=-0.16, p=0.12) and second (r=-0.07, p=0.49) trimesters. Nitric oxide and ADMA also showed a non-significant negative correlation in first (r=-0.08, p=0.46) and in second (r=-0.15, p=0.16) trimesters of pregnancy.
| First trimester | Second trimester | ||||
|---|---|---|---|---|---|
| Positive correlation | p | r | Positive correlation | p | r |
| Placental protein 13 and nitric oxide | 0.04* | 0.21 | Placental protein 13 and nitric oxide | 0.06 | 0.19 |
| Negative correlation | |||||
| Placental protein 13 and asymmetric dimethylarginine | 0.12 | -0.16 | Placental protein 13 and asymmetric dimethylarginine | 0.49 | -0.07 |
| Nitric oxide and asymmetric dimethylarginine | 0.46 | -0.08 | Nitric oxide and asymmetric dimethylarginine | 0.16 | -0.15 |
Table 3. Comparison of correlation of outcome parameters between first and second trimesters of pregnancy.
Discussion
The findings of the present study revealed that emergence of seven preeclampsia cases from the pregnancy group, which amounts to 8.1% in the study population (n=86) which is in line with the data of the National Health Portal of India that reported the incidence of preeclampsia in India is about 8-10% in pregnant women [14].
Preeclampsia is generally characterized by elevation in blood pressure and Proteinuria. In the absence of proteinuria, diagnosis can also be made based on different sign and symptoms. Hence, screening pregnant women at early stage for a diagnosis of onset of preeclampsia is crucial using single or in combination of new biomarkers.
One such biomarker is PP13 that secretes into maternal circulation before6th week of gestation from exosomes or microvesicles and remains even after 2-5 weeks of delivery in maternal circulation. The functional role of PP13 is embryo implantation, provides immune tolerance and increases prostaglandins release which might facilitate in sufficient trophoblast invasion for proper placental development [15]. So the reduced levels of PP13 may probably impair normal placental development. Our previous study findings showed that women with low PP13 levels (477.54 pg/ml) in first trimester of pregnancy developed preeclampsia on follow-up. This data specified that low PP13 levels in first trimester could serve as a marker to identify women with preeclampsia risk. A retrospective study by Romero et al. also gave an idea that low PP13 concentrations in 5-6 weeks of pregnancy were related with the occurrence of preeclampsia [16]. Even though, knowing the possible reason for low concentrations of PP13 in first trimester is challenging, however it can be hypothesized that increased oxidants might influence the expression and secretion of the protein may linked genetically with decreased LGALS13 gene expression.
During follow up in second trimester, elevated levels of PP13 concentration (530.64 ± 69.10 pg/ml) was noticed in preeclampsia compared to normotensive group (518.26 ± 49.33 pg/ml). This steep rise in serum PP13 levels is indicative of increased turnover of the oxidatively stressed syncytiotrophoblast into the maternal circulation. An in vitro experiment by Balogh et al. demonstrated that BeWo cells when exposed to ischemic stress conditions resulted in increased release of PP13 into the culture [17]. Placental hypoxic-ischemia may be a major cause for enhanced trophoblast apoptosis and thus PP13 level increase in maternal circulation in preeclampsia.
In support of the above observation, we measured serum caspase-3 levels in first, second trimesters and in preeclampsia. The basis for caspase-3 selection was on reports from few immunohistological studies that have shown an increased expression of apoptotic marker caspase-3 in villous trophoblasts in preeclampsia [18,19]. The measure of maternal serum caspase-3 in pregnancy and pregnancy complications is scarce. The baseline values of serum caspase-3 were elevated in women who developed preeclampsia compared to normotensive women. A 3-fold elevation of caspase-3 concentration observed in second trimester in women who later developed preeclampsia, suggests placental hypoxic stress with apoptosis might release caspase-3 in placental debris to maternal circulation.
Limited data is available with respect to serum PP13 and its correlation with nitric oxide in humans. Even though, few animal studies have demonstrated infusion of recombinant PP13 to pregnant rats, reduces blood pressure and expands uteroplacental vasculature. However these studies failed to trace the functional role of PP13 with respect to regulation of blood pressure and the pathway responsible for it [20]. Based on this research gap, our current study attempted to evaluate serum PP13 level in humans and extrapolate its possible association with nitric oxide production. Accordingly, the present study results showed positive correlation between PP13 with nitric oxide in both trimesters of pregnancy.
Low levels of serum nitric oxide and increased ADMA levels were noticed in women who developed preeclampsia when compared to the control group. In consistent with our previous report, where the ratio of NO:ADMA was approximately 1:5 in the first trimester of the study group (n-86) [12], similar findings were also observed in second trimester during followup of the study that represent nitric oxide (4.85 nmol/μL) and ADMA (21.06 ng/ml). In cases that developed preeclampsia, the baseline nitric oxide level was 3.71 nmol/μL and ADMA was 21.06 ng/ml in the first trimester in the ratio of 1:7. A striking observation was made in second trimester where the nitric oxide (4.10 nmol/μL) and ADMA (73.43 ng/ml) were in a ratio of 1:18. In the nested case-control study, cases that reported nitric oxide and ADMA from 1:7 to 1:18 from first trimester to second trimester signify the development of preeclampsia around 33 weeks.
Elevated ADMA level in second trimester and in preeclampsia cases suggesting that fetus can also contribute to ADMA concentration. Thereby, we observed a highly significant increase in the serum concentrations of ADMA levels in second trimester (73.43 ± 30.95 ng/ml) compared to the baseline value in the first trimester (21.06 ± 19.42 ng/ml) in first trimester. The three-fold increase could be chronic placental ischemia in preeclampsia leads to oxidative stress which diminishes the activity of the enzyme dimethylarginine dimethylaminohydrolase-2 (DDAH-2) in the placenta signifying the failure of the placenta to degrade ADMA produced by the fetus [21].
To support the above observations, a negative correlation was also observed between nitric oxide and ADMA in preeclampsia but was not found statistically significant; however Mao et al. reported contradictory findings [22].
Conclusion
The nested case-control study conducted revealed measurement of panel of non-enzymatic parameters like PP13, nitric oxide and ADMA, and enzyme caspase-3 assists as good indicators to identify the risk of preeclampsia. Probably PP13 measured in the Indian population is scarce. Fewer studies have performed to study the involvement of biomarkers in placentation and endothelial dysfunction in preeclampsia. Besides, the study also demonstrates a relationship between PP13, ADMA, nitric oxide and caspase-3 which may form the basis of diagnosis of preeclampsia. First and second trimester screening are useful to know the early onset of the disorder associated with the underlying placentation process. Besides PP13, ratio of nitric oxide: ADMA measured during pregnancy is also informative for understanding endothelial function during early pregnancy. Hence, combination of these markers that reflect placentation and endothelial function project their precise screening effectiveness in diagnosing preeclampsia before the onset of symptoms.
Acknowledgements
We thank the authorities of Sri Devaraj Urs Academy of Higher Education and Research for supporting this research study.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Hod T, Cerdeira AS, Karumanchi SA. Molecular mechanisms of preeclampsia. Cold Spring Harb Perspect Med 2015; 5.
- Tannetta D, Sargent I. Placental disease and the maternal syndrome of preeclampsia missing links? Curr Hypertens Rep 2013; 15: 590-599.
- Than NG, Balogh A, Romero R, Karpati E, Erez O, Szilagyi A. Placental protein 13 (PP13)-a placental immuno regulatory galectin protecting pregnancy. Front Immunol 2014; 20: 348.
- Gathiram P, Moodley J. Pre-eclampsia: Its pathogenesis and pathophysiolgy. Cardiovasc J Afr 2016; 27: 71-78.
- Lopez-Alarcon M, Montalvo-Velarde I, Vital-Reyes VS, Hinojosa-Cruz JC, Leanos-Miranda A, Martinez-Basila A. Serial determinations of asymmetric dimthylarginine during pregnancy to predict preeclampsia: a longitudinal study. BJOG 2015; 122: 1586-1592.
- Sharp AN, Heazell AE, Crocker IP, Mor G. Placental apoptosis in health and disease. Am J Reprod Immunol 2010; 64: 159-169.
- Lorente L, Martin MM, Argueso M, Ramos L, Sole-Violan J, Riano-Ruiz M. Serum caspase-3 levels and mortality are associated in patients with severe traumatic brain injury. BMC Neurology 2015; 15: 228.
- Sun DB, Xu MJ, Chen QM, Hu HT. Significant elevation of serum caspase-3 levels in patients with intracerebral hemorrhage. Clin Chim Acta 2017; 471: 62-67.
- Kaya C, Alay I, Guraslan H, Gedikbasi A, Ekin M, Ertaay Kaya S, Oral E, Yasar L. The role of serum caspase 3 levels in prediction of endometriosis severity. Gynecol Obstet Invest 2018; 83: 576-585.
- Cali U, Cavkaytar, Sirvan L, Danisman N. Placental apoptosis in preeclampsia, intrauterine growth retardation, and HELLP syndrome: an immunohistochemical study with caspase-3 and bcl-2. Clin Exp Obstetr Gynecol 2013; 40: 45-48.
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol 1997; 272: 441-463.
- Gadde R, Dayanand C, Sheela S. Maternal serum biochemical indicators of trophoblastic cell and endothelial function at first trimester of pregnancy. Open J Obstetr Gynecol 2018; 8: 867-881.
- Gadde R. Doddaiah D. Rangappa S. Relationship between mean arterial pressure, uric acid and calcium with xanthine oxidase activity and fetal outcome in normotensive and preeclampsia in a nested study. Open J Obstetr Gynecol 2018; 8: 1532-1548.
- Ministry of health and family welfare. NHP India. National Health Portal. Gateway of authentic health information. Preeclampsia 2016.
- Gadde R, Cd D, Sheela SR. Placental protein 13: An important biological protein in preeclampsia. J Circ Biomark 2018; 7: 18494544-18786159.
- Romero R, Kusanovic JP, Than NG. First trimester maternal serum PP13 in the risk assessment of preeclampsia. Am J Obstetr Gynecol 2008; 199: 122.e1-122.e11.
- Balogh A, Pozsgay J, Matko J, Dong J, Kin CJ. Placental protein 13 (PP13/galectin 13) undergoes lipid raft-associated subcellular redistribution in the syncytiotrophoblast in preterm preeclampsia and HELLP syndrome. Am J Obstet Gynecol 2011; 205: 156.e1-14.
- Hsu CD, Polavarapu S, Parton L. PP128. Placental caspase-3 gene polymorphisms is associated with preeclampsia. Pregnancy Hypertens 2012; 2: 308.
- Teimoori B, Yazdi A, Rezaei M, Mohammadpour-Gharehbagh A, Jahantigh D, Salimi S. The association of the placental caspase-3 gene polymorphisms and preeclampsia susceptibility and in-silico analysis. J Cell Biochem 2018; 119: 6756-6764.
- Drobnjak T, Gizurarson S, Gokina NI, Meiri H, Mandalá M, Huppertz B. Placental protein 13 (PP13)-induced vasodilation of resistance arteries from pregnant and nonpregnant rats occurs via endothelial-signalling pathways. Hypertens Pregnancy 2017; 36: 186-195.
- Anderssohn M, Maass LM, Diemert A, Luneberg N, Atzler D, Hecher K, Boger RH. Severly decreased activity of placental dimethylargininedimethylaminohydrolase in preeclampsia. Eur J Obstet Gynecol Reprod Biol 2012; 161: 152-156.
- Mao D, Che J, Li K, Han S, Yue Q, Zhu L. Association of homocysteine, asymmetric dimethylarginine, and nitric oxide with preeclampsia. Arch Gynecol Obstet 2010; 282: 371-375.