Research Article - Biomedical Research (2017) Volume 28, Issue 17
Pioglitazone and low dose of cyclosporin A have synergistic effects in ameliorating rejection after heart transplantation in rats
Qinming Fan, Lei Zheng*, Zhenyu Wei, Maozhou Tian, Xiurong Gong, Pengfei Sun, Yongfeng Lai and Wenyi Liu
Department of Cardiac Surgery, Yantai Yuhuangding Hospital, Shandong University, Yantai, PR China
- *Corresponding Author:
- Lei Zheng
Department of Cardiac Surgery
Yantai Yuhuangding Hospital
Qingdao University, P.R. China
Accepted date: July 14, 2017
Abstract
To investigate the effect of Pioglitazone (Pio) on the rejection reactions that occurs in heart transplantation. A total of 96 rats with heart transplantation were treated with cyclosporin A (CsA) alone, Pio alone, or the combination of both. The percentages of CD25+ and CD28+ T cell in the spleen of rats were measured using flow cytometry. Combined use of Pio and CsA prolonged the survival of transplanted hearts. In addition, pathologic lesions were relieved. Meanwhile, the contents of interleukin-2 in serum and the percentages of CD25+ and CD28+ lymphocytes in the receptor’s spleens were significantly reduced by the combination of Pio and CsA. Of note, the effect of combined use of Pio and CsA was better than that of single use. The present study demonstrates that combined use of Pio and CsA reduces the dose of CsA, ameliorates rejection reactions, and improves the life quality of recipients with heart transplantation.
Keywords
Pioglitazone, Cyclosporin A, Rejection, Lymphokine
Introduction
Heart transplantation is surgical transplantation of hearts performed on patients with advanced congestive heart failure or severe coronary artery diseases [1]. It is widely used in clinical practice, as the most effective therapy for patients with end-stage heart diseases [1]. Due to advances in technology, the survival rate and duration of heart transplant recipients have been significantly increased [2,3]. As the main threat for successful heart transplantation, rejection reaction is the most difficult problem facing all heart transplant recipients [4-6]. Therefore, the development of drugs against rejection reaction attracts more and more attention from researchers.
Peroxisome Proliferator-Activated Receptor (PPAR) is expressed in macrophages, T cells, vascular smooth muscle cells and cardiomyocytes, participating in the regulation of various inflammatory responses [7,8], and the expression of tumor necrosis factor-α and inducible nitric oxide synthase [9]. The agonist of PPAR can improve heart function by inhibiting myocardial hypertrophy and fibrosis via lowering the activity of nuclear factor-kappa B [10]. Pioglitazone (Pio) is a highly selective agonist of PPAR that participates in immune regulation by inhibiting the proliferation of T cells in vitro [11]. Pio also plays important roles in inflammation, arteriosclerosis, and reperfusion injury [12], and promotes inflammatory cell apoptosis [13]. As an outstanding immunosuppressant, cyclosporin A (CsA) enhances the recent survival rate of solid organ transplantats, and becomes the first choice for immunosuppression and anti-rejection after organ transplantation [14-16]. In this study, we investigate the effect of Pio on the symptoms occurring in rat heart transplantation, the survival time of transplanted heart, and immunologic activities.
Materials and Methods
Animals
In the present study, Wistar rats (n=96) were used as donors, and Sprague-Dawley rats (n=96) were used as recipients to build the animal models for intraperitoneal heart transplantation. The recipient rats were randomly divided into 6 groups of 16 rats: Group I (normal control group) was not treated with CsA or Pio; Group II (normal amount CsA group) received intraperitoneal injection of 5 mg/kg CsA at 1 h before surgery and daily after surgery; Group III (normal amount CsA +Pio group) received intraperitoneal injection of 5 mg/kg CsA and intragastric administration of 10 mg/kg Pio at 1 h before surgery and daily after surgery; Group IV (half normal amount CsA group) received intraperitoneal injection of 2.5 mg/kg CsA at 1 h before surgery and daily after surgery; Group V (half normal amount CsA+Pio group) received intraperitoneal injection of 2.5 mg/kg CsA and intragastric administration of 10 mg/kg Pio at 1 h before surgery and daily after surgery; Group VI (Pio group) received intragastric administration of 10 mg/kg Pio at 1 h before surgery and daily after surgery. After surgery, heart beat conditions were determined by careful touches by hand. All animal experiments were conducted according to the ethical guidelines of Qingdao University.
Tissues
On d 3, 7, 14 and 18, four rats in each group were sacrificed for laparotomy. Blood (2 ml) was collected from the inferior vena cava and centrifuged at 1500 rpm for 5 min. The separated serum was mixed with phenylmethanesulfonyl fluoride to prevent degradation, and frozen at -80°C for future ELISA. The excised heart tissues were fixed with 4% paraformaldehyde for pathological examinations. The spleen was made into cell suspension for flow cytometry.
Hematoxylin and eosin staining
After dewaxing by xylene, the sections were treated with high to low concentrations of ethanol, before rinsing with water, acid and ammonia. After washing with flowing water for 1 h, the sections were dehydrated using 70% and 90% ethanol for 10 min each. Then, the sections were stained with eosin for 3 min, before dehydration by pure ethanol and transparency by xylene. Stained slides were cover-slipped with Permount. Finally, the entire HE stained cells were examined under a bright light microscope using 100-400X magnification. The pathological examinations were performed according to the standard by International Society of Heart and Lung Transplantation (ISHLT).
Enzyme-linked immunosorbent assay (ELISA)
The level of interleukin (IL)-2 was measured using the ELISA kit (BD Biosciences, USA). The procedure was carried out according to the manufacturer’s manual. Absorbance at 450 nm was measured using a microplate reader (DJ3022, Potenov Technology Co., Ltd., Beijing, China) within 15 min after stopping the reactions. The concentrations of IL-2 were calculated by plotting standard curves.
Flow cytometry
The spleen was collected from rats after transplantation for the examination of T-lymphocyte subsets. Single-cell suspensions (1 × 106 cells/ml) were stained in cell staining buffer at saturating concentrations according to standard procedures. Cells were stained for CD25+ and CD28+ monoclonal antibodies conjugated to Fluorescein Isothiocyanate (FITC) and Foxp3 monoclonal antibodies conjugated to Phycoerythrin (PE).
Statistical analysis
All statistical analyses were performed with SPSS 18.0 for Windows. Results were expressed as means ± standard deviation for test of normality. If the data conform to normal distribution and the variance is homogeneous, multigroup measurement data were analysed by ANOVA and Dunnett multiple comparison tests and two groups of data were compared by Student’s t-test. If the data does not conform to normal distribution or variance is not uniform, multigroup data were checked by Kruskal-Wallis test and Tamhane's T2 or Dunnett's T3 methods, while two groups of data were examined by Mann-Whitney method. P<0.05 indicated statistically significant differences, while P<0.01 indicated differences that were even more significant.
Results
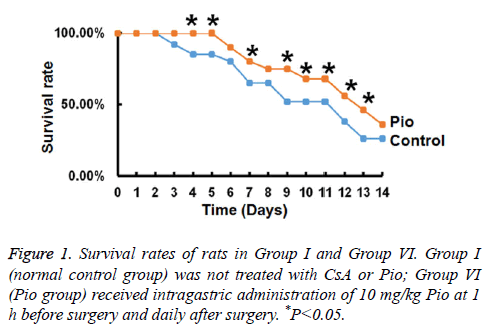
Pio alone prolongs the survival time of transplanted hearts
To investigate whether Pio affects the survival of transplanted hearts, we measured rat survival rate on different days. The data showed that rats in Group VI had significantly higher survival rates compared with those in Group I on the same day (P<0.05). The survival rate in Group VI reached 0 on d 12, while that in Group I reached 0 on day 9 (Figure 1). These data suggest that Pio alone prolongs the survival time of transplanted hearts.
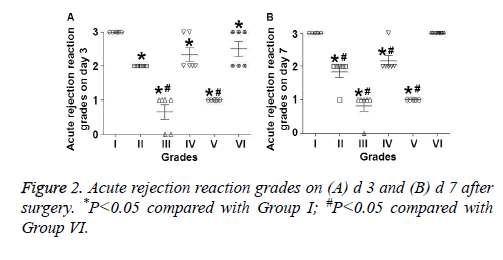
Pio reduces the severity of rejection reactions of transplanted heart, having synergistic effects with CsA
To evaluate the pathology and rejection reactions of transplanted hearts, visual inspection and ISHLT classification were performed. On d’s 3, 7, 14 and 28 after surgery, the size, shape, color and heartbeat power of transplanted hearts in Groups III and V appeared to be better than those in other groups. In addition, use of Pio in Groups III, V and VI ameliorated peritoneal adhesions (data not shown). On d 3 after surgery, the ISHLT grades of rejections in Groups II-V were significantly lower than that in Group I (P<0.01), rejection grade in Group VI was also significantly lower than that in Group I (P<0.05), and rejection grades in Groups III and V were significantly lower than that in Group VI (P<0.05) (Figure 2A). On d 7 after surgery, the ISHLT grades of rejections in Groups II-V were significantly lower than that in Group I (P<0.05), and rejection grades in Groups II-V were significantly lower than that in Group VI (P<0.05) (Figure 2B). On d 14 after surgery, rats in Groups I and VI were all grade 4, most rats in Group II were grade 3A, most rats in Group III were grade 2, most rats in Group IV were grade 3A, and most rats in Group V were grade 1B. On d 28 after surgery, the grade of rejections in Group III was lower than Group II, while that in Group V was milder than Group IV. These results indicate that Pio reduces the severity of rejection reactions of transplanted heart, having synergistic effects with CsA.
Combined use of half normal dose CsA and Pio has the best effect in reducing the levels of IL-2 after heart transplantation surgery
To determine the levels of serum IL-2, ELISA was performed. On d 3 after surgery, the levels of IL-2 in Groups II-V were significantly lower than that in Group I or Group VI (P<0.01), and that in Group VI was significantly lower than that in Group I (P<0.05). On d 7 after surgery, the levels of IL-2 in Groups II-V were significantly lower than that in Group I or Group VI (P<0.01), and that in Group VI was also significantly lower than that in Group I (P<0.01). In addition, the level of IL-2 in Group III was significantly lower than that in Group V (P<0.05). On d 14 after surgery, the levels of IL-2 in Groups III and V were significantly lower than that in Group I (P<0.01), and those in Groups II and IV were also significantly lower than that in Group I (P<0.05). In addition, the levels of IL-2 in Groups II, IV and VI were significantly higher than that in Group V (P<0.05) (Table 1). These results suggest that combined use of half normal dose CsA and Pio has the best effect in reducing the levels of IL-2 after heart transplantation surgery.
| Groups | D 3 | D 7 | D 14 |
|---|---|---|---|
| I | 813.2 ± 28.4 | 935.2 ± 43.5 | 707.6 ± 35.5 |
| II | 603.5 ± 31.8*# | 617.8 ± 35.4*# | 612.4 ± 41.3Δ$ |
| III | 562.4 ± 23.5*# | 573.3 ± 29.6*#a | 570.8 ± 32.2* |
| IV | 623.8 ± 30.5*# | 645.6 ± 28.4*#a | 618.8 ± 34.5Δ$ |
| V | 559.7 ± 21.3*# | 568.4 ± 27.5*# | 536.8 ± 27.8* |
| VI | 729.5 ± 52.4Δ | 737.2 ± 31.0* | 685.5 ± 25.0$ |
| Note: *P<0.01 compared with Group I; #P<0.01 compared with Group VI; ΔP<0.05 compared with Group I; aP<0.05 between each other; $P<0.05 compared with Group V. | |||
Table 1: Concentrations of interleucin-2 on d’s 3, 7 and 14 after surgery (pg/ml) (means ± standard deviation).
Pio alone, or its combination with CsA, delays the appearance of CD25+ and CD28+ T cells in spleen
To detect the spleen lymphocyte subtype markers in cells, flow cytometry was used. On d 3 after surgery, the percentages of CD25+ cells in T lymphocytes in Groups II-VI were significantly lower than that in Group I (P<0.05). On d 7 after surgery, the percentages of CD25+ cells in T lymphocytes in Groups II-VI were significantly lower than that in Group I (P<0.01), and those in Groups II-V were significantly lower than that in Group VI (P<0.05). On d 14 after surgery, no significant differences were observed between any two groups (P>0.05) (Table 2). On d 3 after surgery, the percentages of CD28+ cells in T lymphocytes in Groups II-VI were significantly lower than that in Group I (P<0.01). On d 7 after surgery, the percentages of CD28+ cells in T lymphocytes in Groups II-V were significantly lower than that in Group I or Group VI (P<0.01). On d 14 after surgery, no significant differences were observed between any two groups (P>0.05) (Table 3). These results indicate that Pio alone, or its combination with CsA, delays the appearance of CD25+ and CD28+ T cells in spleen.
| Groups | Day 3 | Day 7 | Day 14 |
|---|---|---|---|
| I | 15.77 ± 3.1 | 33.29 ± 5.2 | 10.79 ± 3.8 |
| II | 7.34 ± 2.4Δ | 9.75 ± 3.6*# | 8.62 ± 2.7 |
| III | 6.44 ± 2.8Δ | 8.65 ± 3.1*# | 11.35 ± 4.3 |
| IV | 7.29 ± 2.5Δ | 12.12 ± 4.3*# | 14.26 ± 2.4 |
| V | 6.22 ± 3.0Δ | 7.48 ± 2.8*# | 7.15 ± 2.3 |
| VI | 8.28 ± 3.5Δ | 20.77 ± 5.6* | 9.02 ± 3.7 |
| Note: ΔP<0.05 compared with Group I; *P<0.01 compared with Group I; #P<0.05 compared with Group VI. | |||
Table 2: The percentage of CD25+ cells in T lymphocytes in spleen cell suspension on d’s 3, 7 and 14 after surgery (%, means ± standard deviation).
| Groups | Day 3 | Day 7 | Day 14 |
|---|---|---|---|
| I | 5.17 ± 1.32 | 7.24 ± 0.98 | 1.75 ± 0.24 |
| II | 1.82 ± 0.21* | 1.93 ± 0.33*# | 1.41 ± 0.32 |
| III | 1.39 ± 0.31* | 1.65 ± 0.27*# | 1.05 ± 0.25 |
| IV | 2.40 ± 0.42* | 2.43 ± 1.02*# | 2.24 ± 0.93 |
| V | 1.06 ± 0.29* | 1.04 ± 0.41*# | 1.03 ± 0.57 |
| VI | 2.46 ± 0.67* | 7.82 ± 1.71 | 1.28 ± 0.43 |
| Note: *P<0.01 compared with Group I; #P<0.01 compared with Group VI. | |||
Table 3: The percentage of CD28+ cells in T lymphocytes in spleen cell suspension on d’s 3, 7 and 14 after surgery (%, means ± standard deviation).
Discussion
The present study showed that rats treated with Pio had better recovery after surgery, longer survival time, and extenuated rejection reactions. In addition, combined use of Pio and CsA showed longer survival time and milder rejection reactions compared with single use of CsA or Pio, demonstrating synergistic effects. IL-2 is a kind of cell factor with extensive biological activities. It enhances non-specific immune response and antigen-specific adaptive immune response by activating cytotoxic T cells and natural killer cells. After heart transplantation, serum levels of IL-2 are gradually elevated as the aggravation of rejection. After the peak time of rejection reactions, serum levels of IL-2 begin to reduce. In the present study, Pio delays the peak time of IL-2, and reduces the concentration of IL-2. Of note, combined use of Pio and CsA has better effect in lowering IL-2 levels compared with using CsA alone.
CD28 molecules can be expressed in all CD4+ T cells or 50% CD8+ T cells. In rejection reactions induced by organ transplantation, the activation of T cells must be triggered by the dual signals presented by antigen-presenting cells. CD28, as a receptor of B7, is mainly expressed on the surface of activated T lymphocytes. The binding of B7 with CD28 transfers activation signals, while the binding of B7 with CTLA4 (CD152) transfers inhibition signals [17]. The binding of B7 and CD28 increases the transcription of interleukin mRNA, enhancing the secretin of IL-2 and the proliferation and differentiation of T cells. In the deficiency of CD28, 90% of IL-2 mRNA can be degraded in 90 minutes, probably because the binding between B7 and CD28 enhances IL-2 gene transcription and stabilizes IL-2 mRNA signals [18]. Rejection reactions in heart transplantation are caused by the direct killing effect by activated CD8+ cytotoxic immune cells. Russell et al. reported that the survival rate of F344 rats with transplanted hearts in CTLA4-LgG treatment group was 64% on d 70, while that in CsA treatment group was 26% [19]. The frequency and severity of arteriosclerosis in CTLA4-LgG treatment group were significantly lower than those in CsA treatment group [19]. Dengler demonstrated that rats treated with CD28 monoclonal antibody had prolonged survival time after heart transplantation [20]. These reports show that CD28 is closely related to rejection reactions after heart transplantation. In the present study, the percentage of spleen CD28+ cells in rats untreated with any drug was significantly increased on d 3 after surgery, and reached peak on d 7. After the acute phase of rejection, the percentage of CD28+ T cells was quickly reduced. Pio delays the appearance of CD28+ T cells in the spleen, while CsA alone or the combination of CsA and Pio significantly inhibited the appearance of CD28+ T cells in the spleen.
Anti-CD25 monoclonal antibody is a novel drug used in organ transplantation. It effectively prolongs the survival time of transplanted organs, especially when it is used together with CsA [21]. CD25 participates in rejection reactions by increasing the expression of myosin heavy chain types I and II antigens, and by affecting the survival of transplanted organ via the regulation of cytokines [22]. Of note, CD25 can cause deviation of the expression of cytokines towards Th1 type, enhancing the expression of IL-4, IL-5, IL-6 and IL-10, and prolonging the survival time of transplanted organs [23].
Immune deviation is demonstrated to be beneficial for the induction and formation of immunologic tolerance. High expression of IL-2, IL-2R and interferon-γ is detected in rejected organs. This phenomenon can be explained by the karyotype differentiation from Th1 to Th2 [24]. However, some other report argue that Th2 immune deviation promotes the occurrence of chronic rejection reactions in transplanted organs with relatively long survival time [25]. Therefore, the long survival of transplanted organs needs the absence of expression of Th1 and Th2 type cytokines. In the present study, higher levels of spleen CD25+ correspond to more severe early rejection rejections. Pio alone, CsA alone or the combination of the two inhibits the early expression of CD25+ in spleen. In conclusion, Pio ameliorates inflammatory responses after heart transplantation in rats, prolongs the survival time of transplanted hearts when used in combination with low dose of CsA, and exerts synergistic effects with CsA in inhibiting rejection reactions.
Acknowledgements
This work was supported by the Provincial Nature Science Foundation of Shandong Province of China (No. Y2007C048).
Disclosures
All authors declare no financial competing interests. All authors declare no non-financial competing interests.
References
- Butler J, Khadim G, Paul KM, Davis SF, Kronenberg MW, Chomsky DB, Pierson RN, Wilson JR. Selection of patients for heart transplantation in the current era of heart failure therapy. J Am Coll Cardiol 2004; 43: 787-793.
- Hetzer R, Albert W, Hummel M, Pasic M, Loebe M, Warnecke H, Haverich A, Borst HG. Status of patients presently living 9 to 13 years after orthotopic heart transplantation. Ann Thoracic Surg 1997; 64: 1661-1668.
- Decampli WM, Luikart H, Hunt S, Stinson EB. Characteristics of patients surviving more than ten years after cardiac transplantation. J Thoracic Cardiovasc Surg 1995; 109: 1103-1114.
- Patel JK, Kobashigawa JA. Should we be doing routine biopsy after heart transplantation in a new era of anti-rejection? Curr Opin Cardiol 2006; 21: 127-131.
- Brunner-La Rocca HP, Sutsch G, Schneider J, Follath F, Kiowski W. Natural course of moderate cardiac allograft rejection (International Society for Heart Transplantation grade 2) early and late after transplantation. Circulation 1996; 94: 1334-1338.
- Mangini S, Alves BR, Silvestre OM, Pires PV, Pires LJ, Curiati MN, Bacal F. Heart transplantation: review. Einstein (Sao Paulo) 2015; 13: 310-318.
- Ozasa H, Ayaori M, Iizuka M, Terao Y, Uto-Kondo H, Yakushiji E, Takiguchi S, Nakaya K, Hisada T, Uehara Y, Ogura M, Sasaki M, Komatsu T, Horii S, Mochizuki S, Yoshimura M, Ikewaki K. Pioglitazone enhances cholesterol efflux from macrophages by increasing ABCA1/ABCG1 expressions via PPARgamma/LXRalpha pathway: findings from in vitro and ex vivo studies. Atherosclerosis 2011; 219: 141-150.
- Wan Y, Evans RM. Rosiglitazone activation of PPARgamma suppresses fractalkine signaling. J Mol Endocrinol 2010; 44: 135-142.
- Gao G, Zhang C, Lv C, Zhang B, Zhang X. Effects of hypothermia resuscitation on expression of peroxisome proliferator activated receptor gamma, inducible nitric oxide synthase and tumor necrosis factor-a genes in lung tissues of rats with hemorrhagic shock. Chinese J Exp Surg 2013; 30: 93-95.
- Simko F. Statins: a perspective for left ventricular hypertrophy treatment. Eur J Clin Invest 2007; 37: 681-691.
- Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J Immunol 2000; 164: 1364-1371.
- Cao Z, Ye P, Long C, Chen K, Li X, Wang H. Protective effects of pioglitazone on heart in ischemia-reperfusion injury rat. Chinese J Clin Pharmacol Ther 2005; 10: 1112-1117.
- Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther 2006; 110: 371-385.
- OKeefe SJ, Tamura J, Kincaid RL, Tocci MJ, ONeill EA. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature 1992; 357: 692-694.
- Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science 1991; 251: 283-287.
- Emmel EA, Verweij CL, Durand DB, Higgins KM, Lacy E, Crabtree GR. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science 1989; 246: 1617-1620.
- Masteller EL, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol 2000; 164: 5319-5327.
- Ba D. Modern technology and applications of immunology. Chinese Peking Union Medical College Press Beijing 1998.
- Russell ME, Hancock WW, Akalin E, Wallace AF, Glysing-Jensen T, Willett TA, Sayegh MH. Chronic cardiac rejection in the LEW to F344 rat model. Blockade of CD28-B7 costimulation by CTLA4Ig modulates T cell and macrophage activation and attenuates arteriosclerosis. J Clin Invest 1996; 97: 833-838.
- Dengler TJ, Szabo G, Sido B, Nottmeyer W, Zimmerman R, Vahl CF, Hünig T, Meuer SC. Prolonged allograft survival but no tolerance induction by modulating CD28 antibody JJ319 after high-responder rat heart transplantation. Transplantation 1999; 67: 392-398.
- Xia J, Jiang X, Huang Y. Effect of CD25 monoclonal antibody on the rejection of heart allograft and the expression of cytokines. Chinese J Exp Surg 2004; 21: 329-331.
- Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, Neylan J, Wilkinson A, Ekberg H, Gaston R, Backman L, Burdick J. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med 1998; 338: 161-165.
- Sollinger H, Kaplan B, Pescovitz MD, Philosophe B, Roza A, Brayman K, Somberg K. Basiliximab versus antithymocyte globulin for prevention of acute renal allograft rejection1, 2. Transplantation 2001; 72: 1915-1919.
- Ankersmit HJ, Moser B, Zuckermann A, Roth G, Taghavi S, Brunner M, Wolner E, Boltz-Nitulescu G. Activation-induced T cell death, and aberrant T cell activation via TNFR1 and CD95-CD95 ligand pathway in stable cardiac transplant recipients. Clin Exp Immunol 2002; 128: 175-180.
- Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis 1999; 5: 285-294.