Research Article - Biomedical Research (2017) Volume 28, Issue 15
Phytochemical, antimicrobial and cytotoxic evaluation of Ziziphus spinachristi (L.) stem bark
Essam Nabih Ads1, Saravanan Rajendrasozhan1, Syed Imran Hassan1,3, Sherif Mohamed Sayed Sharawy1,4 and Jamal Ragheb Humaidi1
1Department of Chemistry, Faculty of Science, University of Hail, Hail, Saudi Arabia
2Faculty of Science, Zagazig University, Zagazig, Egypt
3Sultan Qaboos University, Muscat, Oman
4Faculty of Science, Ain Shams University, Egypt
- *Corresponding Author:
- Essam Nabih Ads
Department of Chemistry
Faculty of Science
University of Hail, Saudi Arabia
Accepted on June 15, 2017
Abstract
Ziziphus spina-christi (L.), commonly called jujube, was evaluated for their phytochemical content in the stem bark as well as the antimicrobial and cytotoxic activities. Z. spina-christi bark was extracted with ethanol and the extract was partitioned between aqueous layer and ethyl acetate layer. The ethyl acetate extract was defatted using diethyl ether and used for GC-MS analysis. The aqueous layer was further extracted with ethyl acetate and drop wise addition ammonia solution to obtain alkaline ethyl acetate extract. Both ethyl acetate extract and alkaline ethyl acetate extract were tested for phytochemical content by qualitative analysis and evaluated for their biological activities such as antimicrobial (by agar well diffusion assay and minimum inhibitory concentration determination) and cytotoxic activities (by MTT assay in cell culture). Phytochemical analysis indicates the presence of tannins, flavonoids, terpernoids, saponin glycosides and alkaloids in Z. spina-christi. Ethyl acetate and alkaline ethyl acetate extracts showed antifungal (against Aspergillus fumigatus, Syncephalastrum racemosum and Geotricum candidum), antibacterial (against Streptococcus pneumoniae, Bacillis subtilis and Escherichia coli), and cytotoxic effects (against colon and breast carcinoma cells). In comparison, alkaline ethyl acetate extract showed significantly (p<0.05) higher antimicrobial activity (against A. fumigatus, S. racemosum and S. pneumoniae) and cytotoxic activities as compared to ethyl acetate extract (IC50 of alkaline ethyl acetate and ethyl acetate extracts=196 and 400 μg/well against colon carcinoma cells and 164 and 397 μg/well against breast carcinoma cells, respectively).
Keywords
Jujube, Stem bark, Cytotoxicity, Antitumor, Antifungal, Antibacterial.
Introduction
Ziziphus genus, belongs to Rhamnaceae family, is traditionally used to cure various human ailments. The root barks of Ziziphus nummularia are reported to have sedative-hypnotic, antipyretic and analgesic purposes [1]. In traditional Chinese medicine, suan zao ren (Ziziphus spinosa) is believed to nourish the heart, and supplement to the liver blood. It is used to treat irritability, insomnia and heart palpitations [2-5]. In Saudi Arabian folk medicine, the leaves of Ziziphus spinachristi (jujube) are used to heal wounds, treat some skin diseases, some inflammatory conditions, sores, against ringworm, fever, gonorrhea, sex diseases and ulcers. The decoction of the bark and fresh fruits is used as a body wash, used to promote the healing of fresh wounds and also used to cure dysentery, bronchitis, cough and tuberculosis [6-9].
Phytochemical investigations on Z. spina-christi have shown that this plant contains many biologically important phytochemicals. From the different species of the genus Zizyphus, peptide and cyclopeptide alkaloids, flavonoids, sterols, tannins, betulinic acid and triterpenoidal saponin glycosides have been isolated and chemically identified [10-18]. Betulic and ceanothic acid, three cyclopeptide alkaloids as well as four saponin glycosides [19] and several flavonoids have been isolated from the leaves of Z. spinachristi [20]. Steroids, β-sitosterol, β-D-glucoside, condensed tannins, and four saponin glycosides have been isolated from the leaves. The free sugars viz. fructose, raffinose, sucrose, glucose, galactose, rhamnose have also been isolated from different parts of the plants [12,21,22]. The butanol extract of the leaves of Z. spina-christi yields four triterpenoidal saponin glycosides and was named as christinin-A, B, C and D, respectively. Christinin-A was the major saponin [19].
The pharmacological screening studies indicated that the aqueous extract of Z. spina-christi root bark has an antinociceptive activity in mice and rats [23] and a central depressant effect in mice [24]. The methanol extract of Z. spina-christi stem bark has antidiarrheal effects in rats [25-28]. Z. spina-christi is also used to relief digestive disorders, obesity, urinary troubles and microbial infections [17,29]. Different parts of the world use Z. spina-christi for skin care [30]. Mohammed et al. studied the aqueous and ethanolic extract of bark of Z. spina-christi that showed the antagonism to acetylcholine induced contraction of rabbit jejunum [31] which confirmed the folkloric use of Z. spina-christi as spasmolytic. The authors also studied the aqueous extract of the bark of Z. spina-christi against various microorganisms to evaluate antibacterial and toxicological activities that showed the usefulness of stem bark for the treatment of wounds, burns, stomach discomfort and urinary tract infections. Arial parts of Z. spina-christi are reported to have cytotoxic effects against human cervix carcinoma and human breast carcinoma cell lines [32].
In view of the medicinal importance of Z. spina-christi, the aim of this study is to extract different fractions from the bark of Z. spina-christi for their antimicrobial and cytotoxic evaluations as well as phytochemical screening.
Materials and Methods
Plant material and extraction
Fresh bark of the Z. spina-christi were collected from the Hail region with GPS coordinates (270 29\05\\N, 410 41\44\\E), (270 30\51\\N, 410 42\01\\E), (270 32\18\\N, 410 41\42\\E), (260 0\21\\N, 400 28\20\\E), Saudi Arabia. The plant was identified and the specimen deposited in the Herbarium of the Department of Biology, University of Hail. Z. spina-christi bark (5.2 kg) was air dried and extracted with absolute ethanol repeatedly (3 times × 20 L). The ethanol extract was filtered, charcoaled and then solvent was evaporated under reduced pressure which yield powdered solid (119.6 g). The ethanol extract was partitioned between aqueous layer and ethyl acetate layer. Ethyl acetate layer was dried and solvent was removed under vacuum to obtain Ethyl Acetate (EA) extract. Aqueous layer was further extracted with ethyl acetate with the drop wise addition of ammonia solution till it become alkaline. The solvent was removed under vacuum to obtained Alkaline Ethyl Acetate (AEA) extract.
Separation and purification of the plant metabolite
Thin Layer Chromatography (TLC) was used to separate plants metabolites from the extracts. Z. spina-christi bark extracts were applied on TLC plates (silica gel G-60 aluminium sheet, Merck, Germany), using CAMMAG Linomat 5 application system and analysed using CAMMAG TLC scanner unit for optimizing the best developing solvent system which separate each crude extract to individual metabolites. TLC plates were activated before use. Developing processes were carried out using the following systems: chloroform: methanol (9.5: 0.5,v/v), toluene: ethyl acetate: formic acid (7: 5: 1, v/v/v) and hexane: ethyl acetate (2: 1, v/v). The compounds were detected from their UV absorbance at 254 and 365 nm or appearance in visible light to detect the characteristics of all spots, i.e. color and Rf value were recorded. The silica gel TLC plates were visualized by placing the plate into iodine vapor.
Phytochemical screening
Z. spina-christi bark extracts were subjected to qualitative phytochemical analysis for the presence of various classes of active chemical constituents such as tannins, saponins, glycosides, flavonoids, alkaloids, terpenes, steroids, etc. using standard procedures [33,34].
GC-MS analysis
The samples were subjected to GC-MS analysis (Thermo Scientific ISQ LT Trace 1310). Injector temperature was 250°C, and column description: TG-SQC GC Column 15 m × 0.25 mm × 0.25 μm. Temperature programming was maintained from 50°C to 290°C with constant rise as follows: Oven Temperature was 50°C increased up to 150°C by rate 7°C/min held for 1 min, increased up to 250°C by rate 5°C/min held for 5 min, increased up to 290°C by rate 10°C/min, held for 2 min. The ion source temperatures and MS line temperature were 300°C. The crudes were injected with a splitless mode. Mass spectra were taken at 70 eV; fragments from 40 to 1000 Dalton. The final confirmation of constituents was made by computer matching of the mass spectra of peaks with the Wiley and National Institute Standard and Technology (NIST) libraries mass spectral database.
Evaluation of antimicrobial activity
Agar well-diffusion assay: All plant extracts were screened against fungi (Aspergillus fumigatus: RCMB 02568, Syncephalastrum racemosum: RCMB 05922, Geotricum candidum: RCMB 05097, Candida albicans: RCMB 05036), gram positive (Streptococcus pneumoniae: RCMB 010010, Bacillis subtilis: RCMB 010067), and gram negative bacteria (Pseudomonas aeruginosa: RCMB 010043, Escherichia coli: RCMB 010052) by using agar well diffusion method. Test organisms were obtained from the Regional Center for Mycology and Biotechnology (RCMB), Cairo, Egypt. The microorganisms were uniformly seeded in sterile malt agar plates for fungi and sterile nutrient agar plates for bacteria. A small well is created (1 cm diameter) in the agar using sterile cork borer and filled with the extracts or standard drugs (100 μL). Standard drugs, such as amphotericin B, ampicillin, and gentamicin were used as positive control. Plates were left in a cooled incubator at 4°C for one hour for diffusion and then incubated at 37°C for tested bacteria and 28°C for tested fungi. Inhibition zones developed due to active antimicrobial metabolites were measured after 24 h of incubation for bacteria and 48 h of incubation for fungi. The clear zone of inhibition in mm was taken as a degree of antimicrobial sensitivity. All experiments were done in triplicate.
Minimum inhibitory concentration (MIC) determination: The MIC of extracts was determined in 96 well microtiter plate using the microdilution broth method [35]. The standard drugs were dissolved in sterile distilled water. The stock solution of the extracts was prepared in DMSO. The dilutions in the test medium were prepared at the required concentration of 512-0.5 μg/ml, and for reference compounds at 64-0.0625 μg/ml. It was established that dilutions of DMSO lacked antimicrobial activity against any of the test microorganisms. The microtiter plates were incubated at 37°C for tested bacteria and 28°C for tested fungi and were readied using microplate reader after 24 h for bacteria and after 48 h for fungi. IC50 was expressed as the concentration (mg/ml) of plant extract necessary to produce a 50% reduction of bacterial culture growth. It was calculated with the calibration curve by linear regression.
Evaluation of cytotoxicity
HCT-116 (colon carcinoma cells) and MCH-7 (breast carcinoma cells) were obtained from VACSERA Tissue Culture Unit. The cells were propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 1% L-glutamine, HEPES buffer and 50 μg/ml gentamycin. All cells were maintained at 37°C in a humidified atmosphere with 5% CO2. For cytotoxicity assay, 0.1 ml of cell suspension containing 10,000 cells was seeded in each well of a 96 well microtitre plate (Falcon, NJ, USA). Fresh maintenance medium containing different dilutions of the test sample was added after 24 h of seeding. Control cells were incubated without test sample. The microtitre plates were incubated at 37°C in a humidified incubator with 5% CO2 for a period of 48 h. Six wells were used for each concentration of the test sample. The number of viable cells was determined using MTT assay [36] by ELISA reader and the percentage of viability was calculated as (1- (ODt/ODc)) × 100% where ODt is the mean optical density of wells treated with the bark extracts and ODc is the mean optical density of untreated cells.
Statistical analysis
Statistical analysis was done by One-Way Analysis of Variance (ANOVA) followed by Duncan’s Multiple Range Test (DMRT).
Results and Discussion
Phytochemical screening
Phytochemical study on Z. spina-christi leaves showed the presence of four saponin glycosides in the butanol extract [19]. As christinin-A was the major saponin glycoside, the butanol extract and chistinin-A were used to evaluate the potential antidiabetic activity and toxicity of Z. spina-christi leaves [37]. Preliminary phytochemical analysis showed the presence of major classes of secondary metabolites such as tannins, alkaloids, flavonoids, cardiac glycosides, etc. in both of the extracts. Saponins, protein and amino acids were absent in both the extracts. Seed extract showed the absence of steroids and terpenoids while their presence was revealed in fruit extract [38]. In our study, phytochemical analysis for the ethanolic extract, ethyl acetate extract and alkaline ethyl acetate extract were carried out. For the ethanolic extract, copious presence of tannins, flavonoids, terpenoids, carbohydrate and saponin glycosides and alkaloids were obtained. While free and combined anthraquinones were absent. Carbohydrates were absent also in the diethyl ether extract (Table 1).
| Chemical Constituents | Ethanol extract | Diethyl ether extract | Ethyl Acetate (EA) extract | Alkaline Ethyl Acetate (AEA) extract |
|---|---|---|---|---|
| Carbohydrate | ++ | - | ++ | ++ |
| Saponin glycosides | ++ | ++ | ++ | ++ |
| Tannins | ++ | - | ++ | ++ |
| Flavonoids | ++ | ++ | ++ | + |
| Terpernoids | ++ | ++ | ++ | ++ |
| Steroids | ++ | ++ | ++ | + |
| Alkaloids | ++ | ++ | ++ | +++ |
| Anthraquinone | - | - | - | - |
+++: Strong intensity reaction; ++: Medium intensity reaction; +: Weak intensity reaction; -: Non-detected.
Table 1. Phytochemical constituents of ethanolic extract fractions of Z. spina-christi stem-bark.
The results of preliminary phytochemical screening confirmed the presence of various classes of secondary metabolites in the ethanol extract of the bark of Z. spina-christi including poly phenols (tannins and flavonoids). Plant polyphenols are important dietary antioxidants because they possess an ideal structural chemistry for free radical scavenging activity. Numerous in-vitro studies have conclusively shown their antioxidant potential in protecting against many diseases [39].
Chemical composition of diethyl ether extract after exthyl acetate of ethanolic extract of Z. spina-christi
GC-MS (Gas Column-Mass Spectrometry) result obtained of molecular weight (m/z) as observed from the spectrum graph ZSC2 are (236), (220), (218), (284 ), (222), (254), (236), (254), (284), (450), (296), (288), (2840), (338), and (284). M/Z for DM2 are (116), (284), (284), (250), and (184). A mass spectrum was produced for each of the compounds represented by peaks in GC chromatogram. The GC-MS instrument used has software that compares the fragmentation pattern of each compound with those in an e-library attached to the software.
The diethyl ether extract showed 13 different compounds, representing 99.96% of the total extract. The identified compounds are listed in Table 2 according to their elution order on TG-SQC GC Column. The major compounds detected were Phytol (53.54%), 14-a-H-Pregna (8.73%), Butyl hydroxytoluene (7.55%). As showed above, 1, 2-15, 16- Diepoxyhexadecane 2-(12-(2-Oxiranyl)dodecyl) oxirane (1.36%), 4, 6, 6-Trimethyl-2-(3-methylbut a-1, 3-dienyl)-3- oxatricyclo (5.1.0.0 (2,4)) octane (1.45%), Retinal (Vitamin A aldehyde) (1.94%) and Menthol, 1’-(butyn-3-one-1-yl)-, (1R, 2S, 5R)-( 2.56%) were also found to be the minor components of the diethyl ether extract.
| RT (min) | Identified compounds | Chromatogram area (%) | MW |
|---|---|---|---|
| 12.53 | 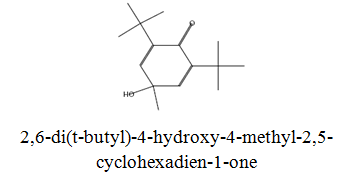 |
2.96 | 236 |
| 13.17 | 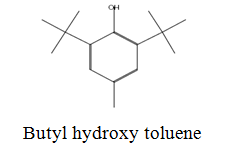 |
7.55 | 220 |
| 18.27 |  |
1.45 | 218 |
| 18.99 |  |
1.95 | 284 |
| 19.15 |  |
2.56 | 222 |
| 19.48 |  |
3.34 | 254 |
| 19.88 | 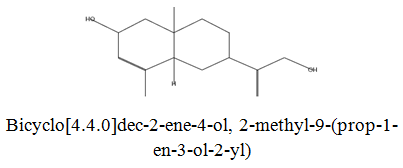 |
4.46 | 236 |
| 21.1 | 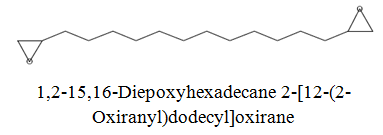 |
1.36 | 254 |
| 22.04 | 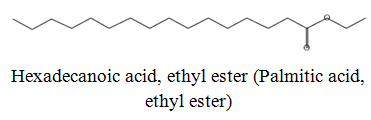 |
3.71 | 284 |
| 23.87 |  |
5.36 | 450 |
| 24.17 | 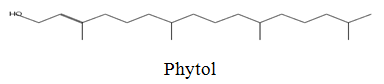 |
53.54 | 296 |
| 24.65 | 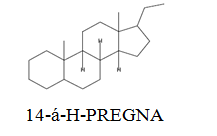 |
8.73 | 288 |
| 25.11 |  |
2.99 | 282 |
Table 2. Chemical composition of diethyl ether extract after ethyl acetate of Z. spina-christi stem-bark.
Antimicrobial activity of Z. spina-christi
The antibiotic resistance has become a serious therapeutic problem [40]. Therefore, the search for novel antimicrobial agents is of the utmost importance. In the present study, the antimicrobial activity of the bark of Z. spina-christi was evaluated against pathogenic bacteria (Streptococcus pneumoniae, Bacillis subtilis, Pseudomonas aeruginosa and Escherichia coli) and fungi (Aspergillus fumigatus, Syncephalastrum racemosum, Geotricum candidum, and Candida albicans). Table 3 shows the mean zone of inhibition beyond well diameter (6 mm) produced against a range of microorganisms using EA and AEA extracts of the bark of Z. spina-christi (1 mg/ml). Both extracts showed antimicrobial activity against these pathogenic microorganisms, except Candida albicans and Pseudomonas aeruginosa. In the presence of AEA extract, the growth inhibition zone is ranged from 13.1 to 21.4 mm for the sensitive bacteria, and ranged from 16.3 to 20.1 mm for fungal strains. But in the presence of EA extract, the growth inhibition zone is ranged from 11.4 to 20.3 mm for the sensitive bacteria, and ranged from 13.2 to 19.4 mm for fungal strains. AEA extract showed significantly (p<0.05) higher antimicrobial activity than EA extract against Aspergillus fumigatus, Syncephalastrum racemosum, and Streptococcus pneumoniae. Strongest antimicrobial activity of the bark of Z. spina-christi was observed against Bacillis subtilis followed by Geotricum candidum. However, since the microbial growth inhibition is not necessarily the indicator of bacterial death, the extension of growth inhibition zone may be due to the bactericidal or bacteriostatic effects of the Z. spinachristi.
| Microorganisms | Zone of inhibition diameter (mm) | ||
|---|---|---|---|
| Ethyl acetate extract | Alkaline ethyl acetate extract | Positive control* | |
| Fungi: | |||
| Aspergillus fumigatus | 14.6 ± 1.2c | 17.2 ± 0.39b | 23.7 ± 1.2a |
| Syncephalastrum racemosum | 13.2 ± 2.1c | 16.3 ± 0.16b | 19.7 ± 0.63a |
| Geotricum candidum | 19.4 ± 1.5b | 20.1 ± 0.58b | 28.7 ± 0.58a |
| Candida albicans | - | - | 25.4 ± 2.1a |
| Gram positive bacteria: | |||
| Streptococcus pneumoniae | 16.2 ± 0.63c | 19.3 ± 0.44b | 23.8 ± 0.63a |
| Bacillis subtilis | 20.3 ± 0.58b | 21.4 ± 0.63b | 32.4 ± 0.58a |
| Gram negative bacteria: | |||
| Pseudomonas aeruginosa | - | - | 17.3 ± 2.1a |
| Escherichia coli | 11.4 ± 2.1b | 13.1 ± 0.25b | 19.9 ± 1.5a |
Data are expressed in the form of mean ± SD, Values not sharing a common superscript differ significantly at p<0.05 (DMRT), -: No activity. *Amphotericin B, ampicillin and gentamicin are used as positive control against fungi, gram positive bacteria and gram negative bacteria, respectively.
Table 3. Antimicrobial activity of ethanolic extract fractions of Z. spina-christi stem-bark.
Agar diffusion technique is considered as a qualitative test for initial screening of antimicrobial activity. It is not an appropriate method for the quantitative evaluation of Minimum Inhibitory Concentration (MIC), as it is impossible to quantify the amount of extract diffused into the agar medium. In this study, MIC of extracts was tested by microdilution broth method. The results in Table 4 confirm that AEA extract has lowest MIC as compared to EA extract against all the sensitive microorganisms. The MIC of AEA extract ranged from 1.95 to 3.90 μg /ml (EA extract: 32.3 to 62.5 μg/ml) against grampositive bacteria, 62.5 mg/ml (EA extract: No activity) against gram-negative bacterium (E. coli), and ranged from 3.90 to 32.3 μg /ml (EA extract: 62.5 to 125 μg/ml) for fungal strains. These results illustrate that a gram-positive bacteria were more susceptible to this extract as compared to gram-negative bacteria. The unique nature of Gram-negative bacteria, such as the presence of two external layers over cytoplasmic membrane and strong hydrophilicity of the outer membranes, explains their strong resistance to Z. spina-christi extracts.
| Microorganisms | MIC (µg/ml) | ||
|---|---|---|---|
| Ethyl acetate extract | Alkaline ethyl acetate extract | Positive control* | |
| Fungi: | |||
| Aspergillus fumigatus | 125 | 15.6 | 0.98 |
| Syncephalastrum racemosum | 125 | 32.3 | 3.9 |
| Geotricum candidum | 62.5 | 3.9 | 0.49 |
| Candida albicans | - | - | 0.49 |
| Gram positive bacteria: | |||
| Streptococcus pneumoniae | 62.5 | 3.9 | 0.98 |
| Bacillis subtilis | 32.3 | 1.95 | 0.49 |
| Gram negative bacteria: | |||
| Pseudomonas aeruginosa | - | - | 15.6 |
| Escherichia coli | - | 62.5 | 3.9 |
MIC: Minimum Inhibitory Concentration, -: No activity. *Amphotericin B, ampicillin and gentamicin are used as positive control against fungi, gram positive bacteria and gram negative bacteria, respectively.
Table 4. Minimum inhibitory concentrations of ethanolic extract fractions of Z. spina-christi stem-bark against microorganisms.
HPLC analysis of bark extracts indicates the presence of saponins in Z. spina-christi. Saponins have a number of pharmacological effects, including the antibacterial effect. The presence of sponins may be responsible for their antibacterial activity [20]. Phytochemical investigation also showed that the bark of Z. spina-christi is rich in tannin and phenolic compounds, which have been shown to possess antimicrobial activities against a number of microorganisms [41,42].
Cytotoxic effects of Z. spina-christi
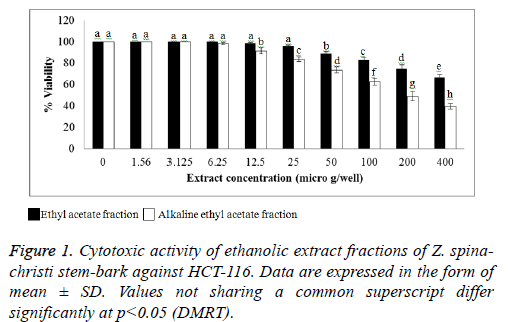
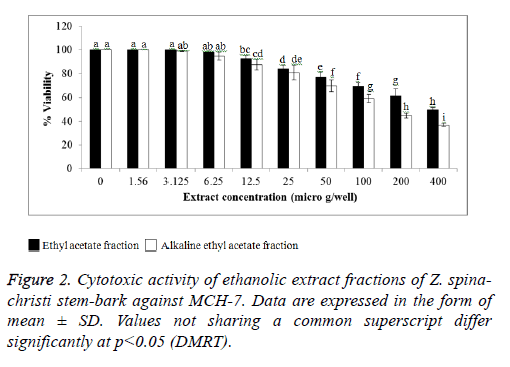
The cytotoxic activity of the EA and AEA extracts of Z. spinachristi (in a concentration of 50-400 μg/well) was investigated in HCT-116 and MCH-7 cell lines. The control cells showed high proliferation that has been taken as 100%. Both extracts induced dose-dependent reduction in the viability of cell-lines (Figures 1 and 2). The IC50 of EA extract in HCT-116 and MCH-7 cells was >400 μg/well and 397 μg/well, respectively. The IC50 of AEA extract in HCT-116 and MCH-7 cells was >196 μg /well and 164 μg /well, respectively. The results indicate that the AEA extract showed higher cytotoxic effect as compared to EA extract. AEA has higher amount of flavonoids and alkaloids as compared to EA extract (Table 1). Flavonoids [43,44] and alkaloids [44] isolated from several plants have shown cytotoxic effects in different cell lines. These phytochemicals may be responsible for the cytotoxic effects of the bark of Z. spina-christi. Phytol, the major phytochemical constituent in the bark of Z. spina-christi (Table 2), is also reported to possess potent cytotoxic effects [45].
Conclusions
The bark of Z. spina-christi has good antifungal, antibacterial and cytotoxic activities. The diethyl ether extract after EA of Z. spina-christi showed 13 different compounds, representing 99.96% of the total extract. The major compounds are phytol (53.54%), 14-á-H-pregna (8.73%) and butyl hydroxytoluene (7.55%). Retinal (Vitamin A aldehyde) (1.94%) and Menthol, 1’-(butyn-3-one-1-yl)-, (1R, 2S, 5R)-(2.56%) were also found to be the minor components.
Ethyl Acetate (EA) extracts of Z. spina-christi rich in saponins and flavonoids showed better antimicrobial and cytotoxic activities as compared to AEA extracts. Further detailed investigations on the isolated compounds are needed to identify the phytoconstituents responsible for the antimicrobial and cytotoxic effects of Z. spina-christi.
Acknowledgements
This work was supported by King Abdulaziz City for Science and Technology (KACST grant No. -ب.ص 35-88 ).
References
- Rauf A, Ali J, Khan H, Mubarak MS, Patel S. Emerging CAM Ziziphus nummularia with in vivo sedative-hypnotic, antipyretic and analgesic attributes 3 Biotech 2016; 6: 11.
- Baumer M. Notes on trees and shrubs in arid and semi-arid regions. Rome FAO Forestry Division 1983.
- Ismail AE. Effect of soil amendments with some hardwood barks on reproduction of Rotylenchulus reniformis and growth of sunflower. Pak J Nematol 1998; 16:137-144.
- National Academy of Sciences. Firewood crops. Nat Acad Press Washington DC 1980.
- Saied AS, Gebauer J, Hammer K, Buerkert A. Ziziphus spina-christi (L.) Willd.: a multipurpose fruit tree. Gene Resources Crop Evol 55: 929-937.
- Irvine RF. Woody plants of Ghana. Oxford University Press 1961; 484.
- Blatter E. Flora arabica. Bishen Singh Mohendra Pal Singh Dehra Dun 1978; 116.
- Duke JA, Ayensu ES. Medicinal plants of China. Reference Publications Inc., Algonac MI 1985.
- Hutchen AR. Indian herbology of North America. Hero Ontario Canada 1973; 382.
- Ikram M, Ogihara Y, Yamasaki K. Structure of a new saponin from Zizyphus vulgaris. J Nat Prod 1981; 44: 91-93.
- Higuchi R, Kubota S, Komori T, Kawasaki T, Pardey VB, Singh JP, Shah AH. Triterpenoid saponins from the bark of Zizyphus joazeiro. Phytochem 1984; 23: 2597-2600.
- Nawwar MM, Ishak MS, Michael HN, Buddrus J. Leaf flavonoid of Zizyphus spina-christi. Phytochem 1984; 23: 2110-2111.
- Han BH, Park MH, Han YN. Cyclic peptide and peptide alkaloids from seeds of Zizyphus vulgaris. Phytochem 1990; 29: 3315-3319.
- Barboni L, Gariboldi P, Torregiani E, Verotta L. Cyclopeptie alkaloid from Zizyphus mucronata. Phytochem 1994; 35: 1579-1583.
- Abu-Zarga M, Sabri S, al-Aboudi A, Ajaz MS, Sultana N. New cyclopeptide alkaloids from Ziziphus lotus J Nat Prod 1995; 58: 504-511.
- Cheng G, Bai Y, Zhao Y, Tao J, Liu Y, Tu G, Ma L, Liao N, Xu X. Flavonoids from Zizyphus jujuba Mill var. spinosa. Tetrahedron 2000; 56: 8915-8920.
- Shahat AA, Pieters L, Apers S, Nazeif NM, Abdel-Azim NS, Berghe DV, Vlietinck AJ. Chemical and biological investigations on Zizyphus spina-christi L. Phytother Res 2001; 15: 593-597.
- Tripathi M, Pandey MB, Jha RN, Pandey VB, Tripathi PN. Cyclopeptide alkaloids from Zizyphus jujube. Fitoterapia 2001; 72: 507-510.
- Mahran GH, Glombitza KW, Mirhom YW, Hartmann R, Michel CG. Novel saponins of Zizyphus spina-christi (L.) Willd cultivated in Egypt. Planta Medica 1996; 62: 163-165.
- Adzu B, Amos S, Wambebe C, Gamaniel K. Antinociceptive activity of Zizyphus spina-christi root bark extract Fitoterapia 2001; 72: 344-350.
- Kamil M, Jayaraj AF, Ahmad F, Gunasekhar C, Samuel S, Habibullah M, Chan K. Pharmacognostic protocols for standardisation of Zizyphus spina-christi. J Pharm Pharmacol 1999; 51: 226.
- Ghazanfar SA. Handbook of Arabian Medicinal Plants. CRC Press 1994; 182.
- Adzu B, Amos S, Wambebe C, Gamaniel K. Antinociceptive activity of Zizyphus spina-christi root bark extract Fitoterapia 2001; 72: 344-350.
- Adzu B, Amos S, Dzarma S, Wambebe C, Gamaniel K. Effect of Zizyphus spina-christi Willd aqueous extract on the central nervous system in mice. J Ethnopharmacol 2002; 79: 13-16.
- Adzu B, Amos S, Amizan MB, Gamaniel K. Evaluation of the antidiarrhoeal effects of Zizyphus spina-christi stem bark in rats. Acta Trop 2003; 87: 245-250.
- Lahlou M, El Mahi M, Hamamouchi J. Evaluation of antifungal and mollusuicidial activities of Moroccan Zizyphus lotus (L.) Desf. Ann Pharm Franc 2002; 60: 410-414.
- Hwang KH, Han YN, Han BH. Inhibition of calmodulin-dependent calcium-ATPase and phosphodiesterase by various cyclopeptides and peptide alkaloids from Zizyphus species. Arch Pharm Res 2001; 24: 202-206.
- Glombitza KW, Mahran GH, Mirhom YW, Michel KG, Motawi TK. Hypoglycemic and antihyperglycemic effects of Zizyphus spina-christi in rats Planta Med 1994; 60: 244-247.
- Nazif NM. Phytoconstituents of Zizyphus spina-christi L. fruits and their microbial activity. Food Chem 2002; 76: 77-81.
- Abbiw DK. Useful plants of Ghana-West African use of wild and cultivated plants. Interm Technol Publ London 1990; 337.
- Mohammed GT, Yesufu HB, Khan IZ, Abdulrahman FI. Effects of aqueous and ethanol extracts of the stem bark of Zizyphus spina-christi L. on isolated rabbit jejunum. J Pharm Biores 2012; 9: 14-19.
- Mohammed GT, Yesufu HB, Abdulrahman FI, Muazul J, Yakubu SI, Sadiq GU, Wazis CH. Antimicrobial and toxicological screening of the aqueous stem bark extract of Zizyphus spina-christi (Linnaeus Desf). J Microbiol Biotechnol Res 2012; 2: 337-342.
- Chothani DL, Patel NM. Preliminary phytochemical screening, pharmacognostic and physicochemical evalution of leaf of Gmelina arborea. Asian Pac J Trop Biomed 2012; 2: 1333-1337.
- Jeyaseelan EC, Jashothan PT. In vitro control of Staphylococcus aureus (NCTC 6571) and Escherichia coli (ATCC 25922) by Ricinus communis L. Asian Pac J Trop Biomed 2012; 2: 717-721.
- Abdel-Zaher AO, Salim SY, Assaf MH, Abdel-Hady RH. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J Ethnopharmacol 2005; 101: 129-138.
- National Committee for Clinical Laboratory Standards. Reference method for broth dilution susceptibility testing of yeast. Appr Standard M27-A Villanova PA 1997.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth 1983; 65: 55-63.
- Alhakmani F, Khan SA, Ahmad A. Determination of total phenol, in-vitro antioxidant and anti-inflammatory activity of seeds and fruits of Zizyphus spina-christi grown in Oman. Asian Pac J Trop Biomed 2014; 4: 656-660.
- Matkowski A, Tasarz P, Szypua E. Antioxidant activity of herb extracts from five medicinal plants from Lamiaceae, subfamily Lamioideae. J Med Plants Res 2008; 2: 321-330.
- Rossolini GM, Arena F, Pecile P, Pollini S. Update on the antibiotic resistance crisis. Curr Opin Pharmacol 2014; 18: 56-60.
- Scalbert A. Antimicrobial properties of tannin. Phytochem 1991; 30: 3875-3883.
- Cowan MM. Plant products as antimicrobial agents Clin Microbiol Rev 1999; 12: 564-582.
- Sak K, Everaus H. Multi-target cytotoxic actions of flavonoids in blood cancer cells. Asian Pac J Cancer Prev 2015; 16: 4843-4847.
- Nwodo JN, Ibezim A, Simoben CV, Ntie-Kang F. Exploring cancer therapeutics with natural products from african medicinal plants, Part II: Alkaloids, Terpenoids and Flavonoids. Anti-Cancer Agents Med Chem 2016; 16: 108-127.
- Pejin B, Kojic V, Bogdanovic G. An insight into the cytotoxic activity of phytol at in vitro conditions Nat Prod Res 2014; 28: 2053-2056.