Research Article - Journal of Parasitic Diseases: Diagnosis and Therapy (2019) Volume 4, Issue 2
Phytochemical analysis and toxicity of Annona muricata stem bark and leaf extracts on Anopheles gambiae larvae.
Paschal Chiedozie Amakiri1,2*, Edith Nonye Nwankwo2, Augustine Chukwuemeka Amakiri3, Chukwudi Micheal Egbuche2, Ikenna Fabian Osuagwu1, Izunna Somadina Okwelogu2, Vivian Onyinyechi Offor2, Chineme T Acha21Chester Medical School, University of Chester, England, UK
2Nnamdi Azikiwe University, Awka, Nigeria
3University of Liverpool, England, UK
- *Corresponding Author:
- Paschal Chiedozie Amakiri
Chester Medical School
University of Chester, England, United Kingdom
Tel: +447721652992
E-mail: 1818021@chester.ac.uk; amakiripaschal42@gmail.com - Nwankwo EN, Okorie PN, Acha CT, et al. Insecticide resistance in Anopheles gambiae s.l Mosquitoes in Awka, Anambra State, Southeast Nigeria. J Mosq Res. 2017;7(5):32-37.
- Cornel AJ, Lee Y, Almeida APG, et al. Mosquito community composition in South Africa and some neighboring countries. Parasit Vectors. 2018;11(1):331.
- Afrane YA, Githeko AK, Yan G. The ecology of Anopheles mosquitoes under climate change: Case studies from the effects of deforestation in East African highlands. Ann N Y Acad Sci. 2012;1249(1):204-10.
- Dhiman S. Is malaria elimination efforts on right track? An analysis of gains achieved and challenges ahead. Infect Dis Poverty. 2019;8(1):14.
- Benelli G, Jeffries CL, Walker T. Biological control of mosquito vectors: Past, present, and future. Insects. 2016;7(4):e52.
- Ghosh A, Chowdhury N, Chandra G. Plant extracts as potential mosquito larvicides. Indian J Med Res. 2012;135(5):581-98.
- Hawkins NJ, Bass C, Dixon A, et al. The evolutionary origins of pesticide resistance. Biol Rev Camb Philos Soc. 2019;94(1):135-55.
- Dang K, Doggett SL, Singham GV, et al. Insecticide resistance and resistance mechanisms in bed bugs, Cimex spp. (Hemiptera: Cimicidae). Parasit Vectors. 2017;10(1):318.
- Ezemuoka LC, Nwankwo EN, Ogbonna CU, et al. Toxicity of the aqueous leaf and Stem-bark extracts of Annona muricata to the 4th instar larvae of Aedes aegypti. J Entomol Zool Stud. 2019;7(4):1047-52.
- Pushpalatha E, Muthukrishnan J. Larvicidal activity of a few plant extracts against Culex quinquefasciatus and Anopheles stephensi. Indian J Malariol. 1995;32(1):14-23.
- Muema JM, Njeru SN, Colombier C, et al. Methanolic extract of Agerantum conyzoides exhibited toxicity and growth disruption activities against Anopheles gambiae sensu stricto and Anopheles arabiensis larvae. BMC Complement Altern Med. 2016;16(1):475.
- Yajid AI, Ab Rahman HS, Wong MPK, et al. Potential benefits of Annona muricata in combating cancer: A review. Malays J Med Sci. 2018;25(1):5-15.
- Khalequzzaman M, Sultana S. Insecticidal activity of Annona squamosa l. Seed extracts against the red flour beetle, Tribolium castaneum (Herbst). 2006;14:107-12.
- Finney DJ, Stevens WL. A table for the calculation of working probits and weights in probit analysis. Biometrika. 1948;35(1-2):191-201.
- Antonio-Nkondjio C, Sandjo NN, Awono-Ambene P, et al. Implementing a larviciding efficacy or effectiveness control intervention against malaria vectors: Key parameters for success. Parasit Vectors. 2018;11(1):57.
- Ngai M, McDowell MA. The search for novel insecticide targets in the post-genomics era, with a specific focus on G-protein coupled receptors. Mem Inst Oswaldo Cruz. 2017;112(1):1-7.
- Nicolopoulou-Stamati P, Maipas S, Kotampasi C, et al. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front Public Health. 2016;4:148.
- Damalas CA, Eleftherohorinos IG. Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health. 2011;8(5):1402-19.
- Mukandiwa L, Eloff JN, Naidoo V. Larvicidal activity of leaf extracts and seselin from Clausena anisata (Rutaceae) against Aedes aegypti. South African J Bot. 2015;100:169-73.
- Elumalai D, Hemalatha P, Kaleena PK. Larvicidal activity and GC-MS analysis of Leucas aspera against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus. J Saudi Soc Agric Sci. 2017;16(4):306-13.
- Mujeeb F, Bajpai P, Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed Res Int. 2014.
- Bagavan A, Rahuman AA, Kamaraj C, et al. Larvicidal activity of saponin from Achyranthes aspera against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res. 2008;103(1):223-9.
- El-Sayed SH, El-Bassiony GM. Larvicidal, biological and genotoxic effects, and temperature-toxicity relationship of some leaf extracts of Nerium oleander (Apocynaceae) on Culex pipiens (Diptera: Culicidae). J Arthropod Borne Dis. 2016;10(1):1-11.
- Raveen R, Kamakshi KT, Deepa M, et al. Larvicidal activity of Nerium oleander L. (Apocynaceae) flower extracts against Culex quinquefasciatus Say (Diptera: Culicidae). Int J Mosq Res. 2014;1(1):38-42.
- Gutierrez PM, Antepuesto AN, Eugenio AL, et al. Larvicidal activity of selected plant extracts against the Dengue vector Aedes aegypti mosquito. Int Res J Biological Sci. 2014;3(4):23-32.
- Kovendan K, Murugan K, Vincent S, et al. Studies on larvicidal and pupicidal activity of Leucas aspera Willd. (Lamiaceae) and bacterial insecticide, Bacillus sphaericus, against malarial vector, Anopheles stephensi Liston. (Diptera: Culicidae). Parasitol Res. 2012;110(1):195-203.
- Mwangi RW, Mukiama TK. Evaluation of Melia volkensii extract fractions as mosquito larvicides. J Am Mosq Control Assoc. 1988;4(4):442-7.
- Kostyukovsky M, Rafaeli A, Gileadi C, et al. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: possible mode of action against insect pests. Pest Manag Sci. 2002;58(11):1101-6.
- Liao M, Xiao JJ, Zhou LJ, et al. Insecticidal activity of Melaleuca alternifolia essential oil and RNA-Seq analysis of Sitophilus zeamais transcriptome in response to oil fumigation. PLoS One. 2016;11(12):e0167748.
Accepted Date: November 20, 2019
Citation: Amakiri PC, Nwankwo EN, Amakiri AC, et al. Phytochemical analysis and toxicity of Annona muricata stem bark and leaf extracts on Anopheles gambiae larvae. J Parasit Dis Diagn Ther. 2019;4(1):8-14.
Abstract
Global efforts to control mosquitoes have relied heavily on the use of synthetic insecticides which have negatively impacted the ecosystem. With the continuous campaign on sustainability and the need to preserve the ecological balance, it has become imperative to develop alternative measures to contain the spread of mosquitoes which are efficient, sustainable and less impactful on the environment. It is in light of these, that laboratory investigations were conducted to determine the phytochemical components present in A. muricata stem bark and leaf extracts. The toxicity effects of various concentrations (200 mg/ml, 100 mg/ml, 50 mg/ml and 25 mg/mL) of A. muricata stem bark and leaf extracts to An. gambiae complex (Diptera: Culicidae) were evaluated by exposing the mosquito larvae at ambient temperature of 29 ± 2°C and 29.5 ± 5% r.h and photoperiod 12:12 light and dark hours. Three replicates of each of the concentrations were used to treat 20 active larvae of An. gambiae in a completely randomized design. Mortality resulting from eclosion inhibition was monitored at 3 hourly intervals for 48 hours, post-treatment and adult emergence were recorded. Data were analysed using log-probit regression and analysis of variance. Results of the qualitative phytochemical analysis revealed the presence of saponins, flavonoids, phenols, cardiac glycosides and alkaloids in both stem bark and leaf extracts. The toxicity result showed that the different concentrations of the extracts resulted in considerable mortality of the larvae. The stem bark and leaf extracts caused significant (p<0.05) mortalities of 100% and 70% respectively at highest concentration of 200 mg/mL while at lowest concentration of 25 mg/mL caused mortalities of 30% and 20% respectively. The percentage inhibition of emergence for stem bark and leaf extracts at highest concentrations were respectively 100% and 70.00%. The LC50 and LC90 are (36.64 and 79.82 mg/mL) and (67.03 and 570.96 mg/mL) respectively for stem bark and leaf extracts. This study suggests that the stem bark and leaf extracts of A. muricata could perhaps be good alternatives to synthetic pesticides that adversely contaminate the environment while also suggesting that the active phytochemicals present in these plant parts could be saponins, flavonoids, alkaloids acting individually or synergistically.
Keywords
Phytochemicals, Toxicity, Anopheles gambiae, Leaf extracts, Pesticides.
Abbreviations
r.h: Relative Humidity; s.s: SENSU Stricto; LC50 and LC90: Lethal Concentration; LT50 and LT90: Lethal Time
Introduction
Mosquitoes are of public health importance because they vector diseases such as malaria [1]. There are about 300 mosquito species globally with Anopheles gambiae being endemic in the tropical regions [2]. Climatic conditions in the tropics favours growth and survival of Anopheles mosquitoes and therefore have led to high prevalence of malaria within the tropics [3]. To reduce prevalence and mortality due to malaria, strategies that either prevents or reduces contact with vectors are encouraged [4]. One such strategy is the eradication of vectors which might include the larval stages [5]. Vector control relies heavily on the use of synthetic pesticides which are not biodegradable and not eco-friendly and therefore pose threats to the environment and non-target organisms [5,6]. Studies have revealed the genetical and physiological resistance of mosquitoes to conventional pesticides [7,8]. Current research efforts are now focused on ecologically tolerable control measures which include the use of inert materials, plant powders, oil and extracts from plants [9,10]. While plants are reported to possess chemicals that may naturally protect them from pests and pathogens, The tropics are endowed with various plants that can be explored in the control of vectors. Crude extracts of Agerantum conyzoides exhibited both larvicidal effect and inhibition of emergence effects on An. gambiae s.s [11]. The effect was attributed to imbalance of developmental hormone [11]. Annona muricata commonly called “soursop” has been reported to have insecticidal activities against Ae. aegypti larvae [9]. More so, amongst the plants investigated for pesticidal activity, Annona muricata showed potential as a repellent against Ae. aegypti [12]. Phytochemicals such as alkaloids were extracted and isolated from Annona squamosa and it showed larvicidal growth regulatory activities against Anopheles stephensi [13]. In view of this, we conducted qualitative and quantitative phytochemical screening on both stem bark and leaf extracts of A. muricata and further evaluated the effects of different concentrations of the plant parts on An. gambiae.
Materials and Methods
Experimental materials
Larval sampling was carried out on stagnant water found within Egbeagu and Okwukwa communities in Amansea. A town in Awka North L.G.A which lies between latitude 6º15’13, 17” N and longitude 6°55’02.93”E. Identification of mosquito larvae was conducted at the research laboratory of the Department of Parasitology and Entomology. Larvae were reared in the laboratory at 29 ± 2ºC and 29.5 ± 5% r.h. The Annona muricata Stem bark and leaves were collected from A. muricata tree found in a farm in the community. The leaves were identified and authenticated at Botany Department, Nnamdi Azikiwe University, Awka, Nigeria. About 500 g of A. muricata leaves and stem bark were dried under shade and pulverized using an electric blender to obtain fine powder. The powdered sample was extracted by soaking 200 g in 600 mL of methanol in a Winchester bottle for 24 hours. This was filtered using Whatman filter paper No. 4 and was kept in a screw cap vial sealed with aluminum foil over the mouth.
This was vigorously shaken to dissolve the material on the solvent; the extract was concentrated over a water bath and was stored at 4ºC for further analysis.
Phytochemical screening
The phytochemical components of the stem and leaf extracts of A. muricata were screened using standard analytical methods to determine Phenols, Total Flavonoids, Tannin, Alkaloids, Saponin, and Cardiac Glycosides.
Toxicity study
The different concentrations of the technical materials obtained from diluting in acetone were used. Appropriate aliquots of 1mL in ml/ml of the formulations were added in plastic cups containing 200 mL of distilled water for both A. muricata stem bark and leaf extracts. Subsequently, 20 wild-caught larvae of An. gambiae were introduced into the cups. Each treatment was replicated 3 times and each bioassay repeated twice. The bioassay was carried out in the laboratory at photoperiod of 12L, 12D, temperature of 29 ± 2ºC and 29.5 ± 5% of relative humidity.
Observation
Mortality/inhibition of emergency assessments was made every 3 hours interval for 24 hours and 48 hours post monitoring for adult emergence.
Dead larvae were counted and recorded. The inability of the larvae to wriggle (moribund) after probing with five forceps was counted as dead. All adults that emerged were counted and recorded.
Data analysis
Log-probit analysis was carried out [14] for determining (LC50 and LC90) and LT50 and LT90 or level of inhibition of emergence (IE%). IE was calculated using the following formula; IE (%)=100-(T × 100/C), where T=percentage survival or emergence in treated batches and C=percentage survival or emergence in the control. One-way Analysis of variance (ANOVA) was also performed on the mortality data in order to determine the level of significance on the effect of both concentration and time.
Results
Phytochemical components
The qualitative phytochemical analysis of the stem bark and leaf extracts showed the presence and absence of certain phytochemicals in these extracts (Table 1).
| Phytochemical component | Plant Parts | |
|---|---|---|
| Stem bark | Leaf extract | |
| Saponin | + | ++ |
| Tannins | ++ | ++ |
| Alkaloids | ++ | ++ |
| Cardiac Glycoside | + | + |
| Phenol | +++ | +++ |
| Flavonoids | + | ++ |
| Anthraquinone | - | - |
Presence of component: +; Absence of component: -
Table 1:Results of the qualitative phytochemical analysis of A. muricata stem bark and leaf extracts.
The phytochemicals present are mainly Saponin, Tannins, Alkaloids, cardiac Glycosides, phenol, and flavonoids. Anthraquinone was not present.
The quantitative phytochemical analysis of the stem bark and leaf extracts showed that Phenols are more in quantity in both the stem bark and leaf extracts than other phytochemicals. More so, Alkaloids and Tannins were also abundant in the two plant extracts. The result showed that the phytochemicals were found to be more in quantity in the leaf extract than in the stem bark extract Table 2.
| Phytochemical components | Plant Parts | |
|---|---|---|
| Stem bark | Leaf | |
| Alkaloids (%) | 2.4 | 1.3 |
| Flavonoids (mg/100 g) | 51.28 | 60 |
| Phenol (mg/100 g) | 412.68 | 667.58 |
| Tannin (mg/100 g) | 35.58 | 3.6 |
| Saponin (%) | 2.2 | 3.6 |
| Cardiac glycosides (%) | 25 | 20 |
Table 2: Quantitative phytochemical analysis of A. muricata Stem bark and leaf extractsables).
Toxicity of A. muricata to the larvae of An. gambiae complex
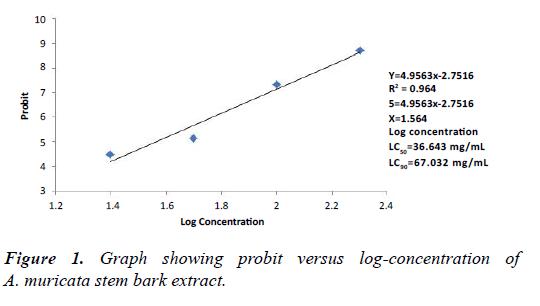
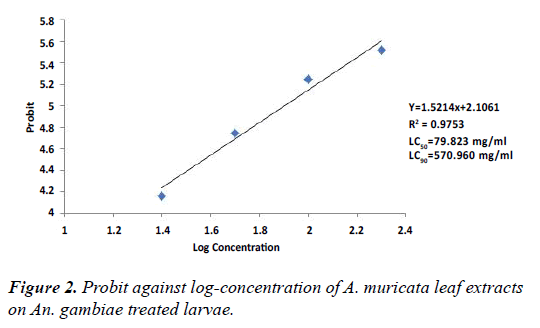
Tables 3 and 4 shows the mortality responses of An. gambiae exposed to different concentrative of A. muricata stem bark and leaf extracts at 48 hours post-treatment. The result showed that A. muricata exhibited significant (p<0.05) levels of toxicity to An. gambiae. There were dose-dependent mortality responses to both plant parts. At the highest concentration of 200 mg/mL of A. muricata stem bark and leaf, mortalities of 100% and 70% were recorded. These tables also show that at the lowest concentration of 25 mg/mL, mortalities of 30% and 20% respectively were recorded. Mortality was also significant (p<0.05). Figures 1 and 2 show the graph of probit against log-concentration of stem bark and leaf extracts of A. muricata. The LC50 and LC90 derived from the graph showed that the stem bark had 36.64 mg/mL and 67.03 mg/mL respectively, while the leaf extract had 79.82 mg/ mL and 570.96 mg/mL respectively.
| Concentration (mg/ml) | Exposure Time (Hours) | Mean ± se | Mortality % | Probit | |||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 24 | 48 | ||||
| 200 | 0 | 2 | 7 | 9 | 12 | 20 | 8.33 ± 2.95 | 100 | 8.72 |
| 100 | 0 | 1 | 5 | 7 | 10 | 18 | 6.83 ± 2.70 | 90 | 7.33 |
| 50 | 0 | 0 | 2 | 3 | 5 | 11 | 3.50 ± 1.69 | 55 | 5.13 |
| 25 | 0 | 0 | 0 | 1 | 2 | 6 | 1.50 ± 0.96 | 30 | 4.48 |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 ± 0.00 | ||
| Mean ± se | 0.00 ± 0.00 | 0.75 ± 0.48 | 3.50 ± 1.56 | 5.00 ± 1.83 | 7.25 ± 2.29 | 13.75 ± 3.22 | |||
| % mortality | 0 | 3.75 | 17.5 | 25 | 36.25 | 68.75 | |||
| Probit | - | 3.23 | 4.07 | 4.33 | 4.65 | 5.49 | |||
Table 3: Mortality effect of different concentration of A. muricata stem bark extract on Anopheles gambiae larvae.
| Concentration (mg/ml) | Exposure Time (Hours) | Mean ± se | % Mortality | Probit | |||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 24 | 48 | ||||
| 200 | 0 | 2 | 5 | 6 | 8 | 14 | 5.83 ± 2.01 | 70 | 5.52 |
| 100 | 0 | 0 | 2 | 2.33 | 4 | 12 | 3.39 ± 1.83 | 60 | 5.25 |
| 50 | 0 | 0 | 0 | 1.33 | 2 | 8 | 1.89 ± 1.27 | 40 | 4.75 |
| 25 | 0 | 0 | 0 | 0 | 1 | 4 | 0.83 ± 0.65 | 20 | 4.16 |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 ± 0.00 | ||
| Mean ± se | 0.00 ± 0.00 | 0.50 ± 0.50 | 1.75 ± 1.18 | 2.42 ± 1.29 | 3.75 ± 1.55 | 9.50 ± 2.22 | |||
| %mortality | 0 | 2.5 | 8.75 | 12.1 | 18.75 | 47.5 | |||
| Probit | - | 3.06 | 3.65 | 3.83 | 4.11 | 4.94 | |||
Table 4: Mortality effect of different concentrations of A. muricata leaf extract on Anopheles gambiae larvae.
Effect of exposure time of A. muricata stem bark and leaf extracts on An. gambiae treated larvae
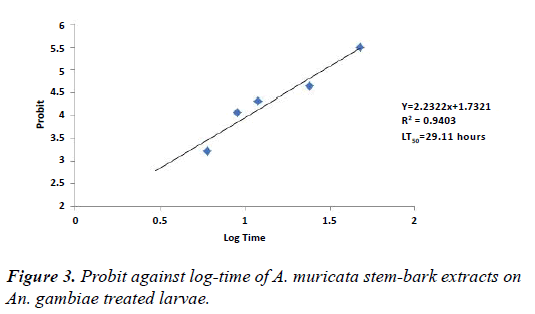
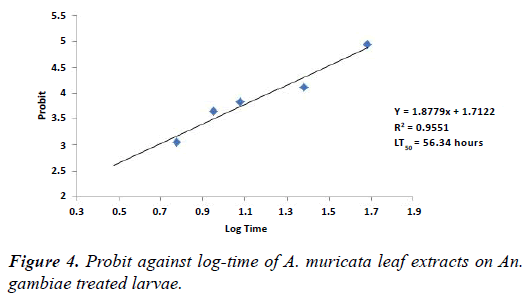
From the results (Tables 3 and 4), exposure time has an effect on various concentrations of A. muricata stem bark and leaf extracts on An. gambiae treated larvae. After 6 hours exposure of the extracts, mortalities were respectively 3.75% and 2.50%. However, as time progressed to 48 hours, mortalities were 68.75% and 47.50% for stem bark and leaf extracts respectively. Figures 3 and 4 show the graph of probit against log-time of stem bark and leaf extracts of A. muricata. LT50 of stem bark and leaf extracts derived from the graph were 29.11 hours and 56.34 hours respectively. This shows that exposure time was significant (p<0.05).
Effect of the plant extracts on adult emergence of An. gambiae
The adults of An. gambiae that emerged from the larvae treated with stem bark and leaf extracts of A. muricata is presented in Tables 5 and 6. The results showed that at the highest concentration of 200 mg/mL of stem bark and leaf extracts, percentage adult emergence was 0% and 30% respectively. However, at the lowest concentrations of these two plant extracts, the stem bark extract had percentage emergence of 70% while the leaf extract had percentage emergence of 80%. These were not significantly different (p>0.05) when compared with control which recorded 100% emergence. The time of exposure also had effect on the number of emerged adults. At 72 hours of exposure, only 1.25 adults emerged in both stem bark and leaf extracts respectively (Tables 5 and 6). At 168 hours of exposure up to 31.25% of the adult emerged from water treated with stem bark extract while 25.0% emerged from water treated with leaf extract.
| Concentration (mg/ml) | Exposure Time (Hours) | % Emergence | |||||
|---|---|---|---|---|---|---|---|
| 72 | 96 | 120 | 144 | 168 | Mean ± se       | ||
| 200 | 0 | 0 | 0 | 0 | 0 | 0.00 ± 0.00 | 0 |
| 100 | 0 | 0 | 1 | 2 | 2 | 1.00 ± 0..45 | 10 |
| 50 | 0 | 0 | 2 | 4.00. | 9 | 3.00 ± 1.67 | 40 |
| 25 | 1 | 4 | 6 | 8 | 14 | 6.60 ± 2.18 | 70 |
| Control | 3 | 6 | 8 | 10 | 20 | 9.40 ± 2.89 | 100 |
| Mean ± se | 0.25 ± 0.25 | 1.00 ± 1.00 | 2.25 ± 1.32 | 3.50 ± 1.71 | 6.25 ± 3.22 | ||
| %emergence | 1.25 | 5 | 11.25 | 17.5 | 31.25 | ||
Means of three replicates (± s. e); LSD for concentration: 5.29; LSD for time: 5.41; p value for concentration: 0.007; p value for time: 0.195
Table 5: Adult emergence of An. gambiae treated larvae on various concentration of A. muricata stem bark extract.
| Concentration (mg/ml) | Exposure Time (Hours) | Mean ± se | % Emergence | ||||
|---|---|---|---|---|---|---|---|
| 72 | 96 | 120 | 144 | 168 | |||
| 200 | 0 | 0 | 0 | 1 | 3 | 0.80 ± 0.58 | 30 |
| 100 | 0 | 0 | 0 | 1 | 4 | 1.00 ± 0.77 | 40 |
| 50 | 0 | 1 | 2 | 3.00. | 5 | 2.20 ± 0.86 | 50 |
| 25 | 1 | 1 | 3 | 3 | 8 | 3.20 ± 1.28 | 80 |
| Control | 2 | 4 | 5 | 7 | 10 | 5.60 ± 1.36 | 100 |
| Mean ± se | 0.25 ± 0.25 | 0.50 ± 0.28 | 1.20 ± 0.75 | 2.00 ± 0.57 | 5.00 ± 1.08 | ||
| %emergence | 1.25 | 2.5 | 6.25 | 10 | 25 | ||
Means of three replicates (± Â s. e); LSD for concentration: 3.00; LSD for time: 2.00; p value for concentration: 0.021; p value for time: 0.001
Table 6: Adult emergence of An. gambiae treated larvae on various concentration of A. muricata leaf extract.
Percentage inhibition of emergence (IE%)
The result of inhibition of the emergence of An. gambiae treated with stem bark and leaf extracts of A. muricata is represented in Tables 7 and 8. The results showed that at the highest concentration of 200 mg/mL of stem bark and leaf extracts, inhibition of emergence (IE%) were 100% and 70% responsively. However, at the lowest concentration of the two plant extracts, the stem bark extract had inhibition of emergence (IE%) of 30% while the leaf extract had inhibition of emergence (IE%) of 20%.
| Concentration (mg/ml) | Percentage Emergence (%) | Inhibition of Emergence (IE)% |
|---|---|---|
| 200 | 0 | 100 |
| 100 | 10 | 90 |
| 50 | 40 | 60 |
| 25 | 70 | 30 |
| Control A (Acetone) | 100 | 0 |
| Control B (Water) | 100 | 0 |
Table 7: Percentage emergence and inhibition of emergence of A. muricata stem bark extract on An. gambiae complex.
| Concentration (mg/ml) | Percentage Emergence (%) | Inhibition of Emergence (IE)% |
|---|---|---|
| 200 | 30 | 70 |
| 100 | 40 | 60 |
| 50 | 50 | 50 |
| 25 | 80 | 20 |
| Control A (Acetone) | 100 | 0 |
| Control B (Water) | 100 | 0 |
Table 8: Percentage emergence and inhibition of emergence of A. muricata leaf extract on An. Gambiae.
Discussion
The phytochemical analysis and toxicity of stem bark and leaf extracts of Annona muricata to larvae of Anopheles gambiae complex were studied in the laboratory. The studies have shown that each plant extract contained at least one of the important phytochemical constituents and that the extracts could be used as independent control method in reducing the impact of mosquitoes. Identification of various plant extracts that have larvicidal potential against mosquitoes could be of advantage in reducing the problem of resistance and environmental contamination [6].
Mosquitoes in their larval stage are attractive targets for pesticides because mosquitoes breed in water, which makes it easy to control them in this habitat [15,16]. The use of conventional pesticides in the water sources, however, introduces many risks to people and the environment [17,18].
Therefore, natural pesticides especially those derived from plants are more promising in this aspect.
Many plant chemicals produce larvicidal effects. This agreed with [19,20] who reported some paramount bioactive constituents of plants that have larvicidal activity. Also, it has been reported that leaf extracts of Aegie marmelos contained saponins, flavonoids, tannins and other phytochemicals [21]. This finding is in line with the present study that reported that the stem bark and leaf extracts of Annona muricata contained these phytochemical components such as flavonoids, alkaloids, saponins and known for their toxicity to harmful insect. Saponins isolated from Achyranthes aspera possess larvicidal activity against Ae. Aegypti and Cu. quinquefasciatus [22]. Also, flavonoids isolated from seed extracts of A. squamosa were reported to be effective as insecticides against the red flour beetle [13]. It is therefore suggested that the pesticidal actions of A. muricata stem bark and leaf extracts may be attributed to the presence of the phytochemicals present in the plant (flavonoids, tannins, saponins, and alkaloids).
The different responses induced by phytochemicals on various species of mosquitoes could have been influenced by extrinsic and intrinsic factors such as the plant species, plant part, the solvent used for extraction, the geographical location where the plants were grown and the methods employed for extraction [23]. Phytochemicals can be extracted from either the whole plant or specific parts of the plants depending on the activity of the derivatives [6]. Plants accumulate bioactive chemicals differentially in the various parts of the plant such as leaves, fruits, flowers, roots and bark and the effectiveness of chemicals derived from them varies with the mosquito species [6]. The isolated compound saponin from ethyl acetate extract of Achyranthes aspera was effective against the larvae of Ae. aegypti and Culex quinquefasciatus with LC50 value of 18.20 and 27.24 ppm respectively [22]. In the present study, the crude extracts of stem bark and leaf of A. muricata caused mortality of 100% and 70% respectively at 200 mg/mL and caused inhibition of emergence of 100% and 70% respectively.
Crude extracts of many plants showed larvicidal activity against Anopheles mosquito and other mosquito species and the results of the present study were comparable with those reports. For instance, it was reported that ethanolic leaf extract Nerium oleander exhibited larvicidal activity against Ae. aegypti with LC50 value of 197.97 mg/L [24]. More-so, aqueous leaf extract and stem bark extracts of Annona muricata were reported to have resulted in 65% and 87.1% mortality of Aedes aegypti at the highest concentrations [9] which were consistent with the present study and suggest that the stem bark exerts better larvicidal effect than the leaf extracts. In the present study also, the crude extracts of the stem bark and leaf of A. muricata exhibited larvicidal activity against the larvae of An. gambiae with LC50 and LC90 values of (39.5 mg/mL and 75.12 mg/ml) and 8.02 and 807.2 mg/mL respectively. In another study, [25] reported LC50 and LC90 of 10 mg/mL and 40 mg/mL respectively after 48 hours of exposure of Ae. aegypti to plant leaf extracts. Recent studies on the larval and pupal mortality of An. Stepehensi after treatment with methanolic extract of clerodendron inerme leaf extract showed 22% mortality of larvae at 20 ppm [26]. This finding contradicts the present study where the leaf extract of A. muricata at 200 mg/mL caused up to 70% of mortality of larvae of An. gambiae. In this study the toxic effect of the extracts was high, as the mortality and the rate of inhibition of emergence increased with increasing concentration and time of exposure. In another study, [27] it was observed that fraction of melia volkensi fruit kernel extracts had growth inhibition activity at very low concentrations, whereas two other fractions had acute toxic effects on the mosquito larvae. Thus, in the present studies, only a few surviving larvae were left in some treated samples, and they were able to mature to adult stage.
It was also reported by Pushpalatha E [10] that leaf extracts of vitex negundo at very low concentrations had larvicidal activity against Cu. quinquefasciatus and An.stephensi and also extended the derivation of larval pupation.
The botanical pesticide, A. muricata has proved to be very effective in mosquito larval control as demonstrated in the present studies. The activity extended over a longer period and its effectiveness increased with increasing exposure time. The extracts probably exhibited their effects on the mosquito larvae as growth regulators, as well as blocking the respiratory organs. It has been reported that some plant essential oil block the octopamine neuroreceptors that regulate the movement, heart rate, behaviour, metabolism, and pupation of insects [28,29]. Thus, the regulatory activity of A. muricata plant parts was exhibited in the mortality of the larval and inhibition of their eclosion into adult stage. Further investigations are therefore needed to elucidate their activity against a wide range of all stages of mosquito species and if possible, to isolate the active ingredient(s) of the extracts responsible for larvicidal activity.
Conclusion
The study on the phytochemical analysis and toxicity of methanolic extracts of stem bark and leaf of A. muricata to An. gambiae complex showed that the stem bark and leaf extracts of A. muricata contain alkaloids, flavonoids, phenol, saponins, and tannins. These compounds are known to possess insecticidal and larvicidal activities on insects and other animals.
The crude extracts of A. muricata plant parts showed larvicidal activity to An. gambiae complex larvae which are manifested by high percentage mortality and inhibition of emergence compared to those in the control groups. The mortality of mosquito larvae between the various concentrations of the plant extracts and the control group is also significantly different. Furthermore, the stem bark of A. muricata extract shows the most effective larvicide compared to the leaf extract with percentage mortality of 100% at the highest concentration of 200 mg/mL and 68.75% after 48 hours of exposure.
The present study therefore, demonstrate that A. muricata could be used as mosquito larvicide for the control of An. gambiae complex. This would help to reduce the mortality and morbidity due to malaria transmitted by this mosquito. It would be necessary to increase campaign on the growth of this plant in all ecological zones of Nigeria, as an invaluable tool for the control of mosquito vector.
Acknowledgement
We are grateful to Prof Stanley Udedi and Mr. KK Asogwa of the Stepping Stone R and D laboratory for allowing us to use their research laboratory for the phytochemical analysis. We are also grateful to the staff of the Parasitology and Entomology Department of the Nnamdi Azikiwe University, Awka, Nigeria and the herbarium curator of the Department of Botany Nnamdi Azikiwe University, Awka, Nigeria.