Research Article - Journal of Clinical Ophthalmology (2021) Volume 5, Issue 1
Peripapillary retinal nerve fibre layer thickness, ganglion cell thickness and foveal thickness in school going children as measured by spectral domain optical coherence tomography.
Dilon D. Noronha*, Norman B Mendonca
Department of Ophthalmology, Father Muller Medical College, Mangaluru, India
- Corresponding Author:
- Dr. Dilon Noronha
Department of Ophthalmology
Father Muller Medical College
Mangaluru
India
E-mail: dilonnoronha@gmail.com
Accepted date: 05 January, 2021
Citation: Noronha DD, Mendonca NB. Peripapillary retinal nerve fibre layer thickness, ganglion cell thickness and foveal thickness in school going children as measured by spectral domain optical coherence tomography. J Clin Ophthalmol 2021;5(1):334-341
Abstract
Purpose: The study was conducted to compile a normative database for SD-OCT measurements of foveal thickness, Ganglion cell thickness as well as peripapillary RNFL thickness in paediatric age of 7-15 years and to analyse the correlation of such values with age, gender and refractive error (SE)
Methods: The study enrolled 200 eyes of 100 children (47 males and 53 females) that attended the Department of ophthalmology, Father Muller Medical College, Mangalore from September 2016-2017. A complete ophthalmological examination was done including cycloplegic refraction and fundus evaluation with 90 D lens. Spectral domain OCT was performed using CIRRUS HD OCT after pupillary dilatation. Signal strength of 6 or higher was considered acceptable.
Results: 100 children were included in this study, 47 males and 53 females. The mean age of patients was 12.07 ± 2.46 SD years. Average spherical equivalent (SE) was -0.301 ± 0.72 SD dioptres in the right and -0.29 ± 0.69 SD dioptres in the left eye. The mean global RNFL thickness was 93.395 μm and the mean GCC thickness was 78.42 μm. Both were marginally higher in females (93.1887, 79.7547) compared to males (91.9787, 77.2979) with p=0.67 and 0.33 respectively. RNFL thickness was maximum in the inferior quadrant (122.265 μm) followed by superior (121.305 μm), nasal (69.325 μm) and temporal quadrants (62.485 μm). The mean CFT in this study was 234.325 μm and the mean MV was 9.697 μm. It was marginally higher in males compared to females.
Conclusion: RNFL thickness followed a normal distribution. RNFL, foveal and GCC thickness varied marginally with gender and had no significant correlation with age and refractive error. The normative data from this study could serve as reference for further studies on paediatric glaucoma using newer imaging modalities.
Keywords
Retinal Nerve Fibre Layer (RNFL), Ganglion cell complex, Central foveal thickness, Cirrus HD OCT.
Introduction
Optical Coherence Tomography (OCT) is a non-invasive, noncontact technique that visualizes the retina and has increasingly been used in ophthalmology [1,2]. It is a new imaging technology which is based on the Laser Interferometry principle. Image capture is fast and painless.
Retinal thickness can be measured accurately by OCT due to its high depth resolution (10 microns). Comparison of OCT and histological images from prototype devices has shown good correlation between real measurements of Retinal Nerve Fibre Layer (RNFL) thickness and OCT estimates.
The third-generation instrument, Stratus OCT (Carl Zeiss Meditec, Dublin, CA, USA), relies on time-domain technology (TD-OCT). This technology recently was superseded by new instruments that use spectral-domain technology (SD-OCT), such as Cirrus OCT (Carl Zeiss Meditec).
Spectral domain OCT (SD-OCT) provides approximately twice the axial resolution and 45 to 100 times the scanning speed and can reveal the three-dimensional configuration of the retina in comparison with time-domain OCT (TD-OCT) [3].
SD-OCT significantly increases the amount of data acquired during each session; the motion artefacts are significantly reduced; and better repeatability and reproducibility and an increased signal-to-noise ratio are achieved compared with TDOCT. Cirrus HD-OCT (Cirrus Version 6.0; Carl Zeiss Meditec, Dublin, CA) is a commercially available SD-OCT with a scan speed of 27,000 axial scans per second and an axial resolution of 5 μm [4].
The diagnosis and follow-up of children with an ocular disease is more difficult than that of adults because of the challenge in obtaining reliable and reproducible visual examinations. Important diagnostic tools used in adults such as visual fields, require their cooperation. For children, such tools are often impractical because the results are unreliable, and hence difficult to interpret However, OCT provides objective measurements of the affected structures. Generally, children older than 3 or 4 years of age can cooperate sufficiently. Macular measurements are even easier to obtain than those of the optic nerve, making OCT particularly suitable for use with uncooperative children or those with poor fixation.
SD-OCT is progressively being used to evaluate paediatric macular diseases, childhood glaucoma, and non-glaucomatous optic neuropathies. Additionally, it is used for monitoring changes in the progression of the disease and assessing the efficacy of current and novel treatments for eye diseases in paediatric population.
Materials and Methods
Period of study and source of data
The present study was conducted in the Department of Ophthalmology, Father Muller Medical College and Hospital, Mangalore, for a period of one year (September 01, 2016-September 01, 2017). All School going children attending the ophthalmology out-patient department at Father Muller Medical College Hospital for their routine eye examination were included in this study.
Study type: An institutional cross-sectional observational study.
Selection criteria
Inclusion criterion:
• Both sexes with ages ranging from 7-15 years.
• Children who were born at term (>37 weeks gestational age) and with normal birth weight (>2500 g).
• Children with Best Corrected Visual Acuity (BCVA) of 6/6 (on the Snellen scale) for both eyes, refractive error (in SE) within ± 4.00 dioptres.
• IOP<21 mmHg in both eyes, cup-to-disc (C/D) ratio<0.4, and C/D ratio asymmetry <0.2 between the 2 eyes.
• Patients with no history of intraocular surgery, neurological disease, retinal disease, glaucoma, Amblyopia or Nystagmus.
Exclusion criteria:
• Parents not willing to provide informed consent.
• Patients not cooperative for SD-OCT examination.
• High refractive error (in SE) exceeds ± 4.0 D and/or astigmatism exceeding 3.0 D.
• Intraocular pressure (IOP) >21 mm Hg and eye pathology that may affect OCT measurements.
• Children with history of ocular abnormalities like Amblyopia, strabismus.
• Family history of optic nerve or retinal pathologies.
Statistical analysis
• All data collected in the study were sorted, coded, and entered in an excel sheet and then transferred to the SPSS v. 23 software program for data management and analysis.
• The outcome parameters assessed were average peri papillary RNFL thickness, macular thickness and ganglion cell complex thickness.
• The descriptive data were analysed using mean, percentage, standard deviation. Inferential statistics were analysed using Pearson’s correlation coefficient and Chi-square test.
• Unpaired t-test was used to compare between two groups (male v/s female, right eye v/s left eye).
• Correlation and regression analysis were done to assess the relationship between RNFL, foveal thickness and GCC thickness with clinical parameters (age, gender and refractive error).
• A p-value of 0.05 or less was considered to be statistically significant.
Methodology
• A total of 100 children aged between 7-15 years presenting to Department of Ophthalmology in Father Muller medical college, Mangalore from September 2016 to September 2017 were included in the study.
• A written informed consent to participate in the study was obtained from parents of minor subjects after explaining the imaging modality to them and to the child.
• A brief history and comprehensive clinical examination was carried out in every patient including Best corrected Visual acuity (BCVA), slit-lamp examination, Intraocular Pressure (IOP) assessment (Goldmann applanation tonometry wherever feasible, I care measurement or palpation in less cooperative patients), Extra ocular motility assessment, Cycloplegic retinoscopy and dilated fundoscopy using direct and indirect Ophthalmoscopy. Visual acuity testing was performed using Snellen’s charts.
• Cirrus HD-OCT (Carl Zeiss, Dublin, California, USA) device was used to obtain high-definition images. All imaging was performed by the same experienced ophthalmic photographer after pupillary dilatation.
• The protocol used for RNFL assessment was the optic disc cube where a 3.46 mm circular scan is placed around the optic disc and the information about peri papillary RNFL thickness is obtained where by 27,000 A scans are acquired per second. The peripapillary RNFL thickness parameters automatically calculated by the Cirrus software and evaluated in this study included average/full circle thickness –RNFL-FC (360° measure), temporal quadrant thickness, RNFL-T, superior quadrant thickness, RNFL-S, nasal quadrant thickness, RNFL-N, inferior quadrant thickness, RNFL-I.
• Three such circular scans were performed successively. The average of the 3 scans was used in the analysis. Mean RNFL thickness in micrometers along the whole circle circumference, four quadrants, 12’o clock hours were obtained.
• Macular cube 512 × 128 and optic disc cube 200 × 200 was utilized to assess macular and peripapillary RNFL thickness respectively.
• The Ganglion cell complex (GCC) maps were based on macular protocol centred on fovea with a cube of 512 × 128 with automated measurement of GCC and internal limiting membrane. Signal strength of 6 or higher was considered acceptable.
Results and Observations
A total of 100 children were enrolled in this study. Out of these 100 participants, 47 were males while 53 were females. The age of the patients in this study ranged from 7 to 15 years with the mean of 12.07 ± 2.46 SD years as shown in Figures 1 and 2. 100 right eyes and 100 left eyes were analysed.
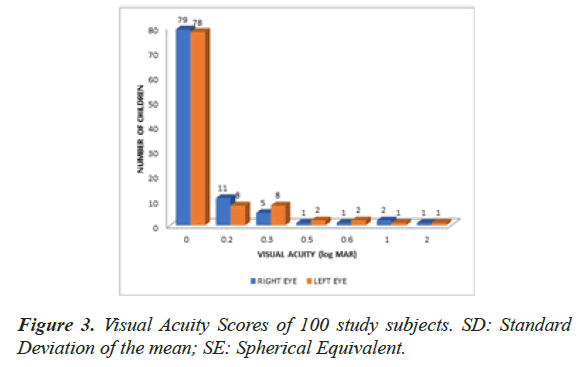
The Visual acuity of children ranged from log MAR 0 to log MAR 2 with a mean refractive error(SE in dioptres) of -0.301 ± 0.72 SD dioptres in the right eye and -0.29 ± 0.69 SD dioptres in the left eye as shown in Figure 3. The mean global RNFL thickness (RNFL-FC) was 93.395 μm. The RNFL thickness was maximum in the inferior quadrant (RNFL-I) 122.265 μm followed in order by superior (RNFL-S) 121.305 μm, nasal (RNFL-N) 69.325 μm and temporal (RNFL-T) 62.485 μm as depicted in Tables 1 and 2.
| Variable | Mean | SD | 95% CI | IQ Range | ||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | |
| RNFL-FC | 94.17 | 92.62 | 8.96 | 14.05 | 1.75 | 2.75 | 88-100 | 86-100 |
| RNFL-I | 123.83 | 120.7 | 18.69 | 26.44 | 3.66 | 5.18 | 114-135 | 112-134 |
| RNFL-S | 119.68 | 122.9 | 18.98 | 19.95 | 3.72 | 3.91 | 112.75-128.25 | 112-136.5 |
| RNFL-N | 70.95 | 67.7 | 13.11 | 18.48 | 2.56 | 3.62 | 62-78 | 58.75-74.25 |
| RNFL-T | 62.73 | 62.24 | 11.39 | 13.13 | 2.23 | 2.57 | 54-70 | 55.75-68 |
Table 1: Mean global RNFL thickness and distribution of RNFL in each quadrant.
| Gender | N | Mean | Std. Deviation | t | df | p-value | |
|---|---|---|---|---|---|---|---|
| Average RNFL(µm) right | M | 47 | 94.4468 | 8.63212 | 0.289 | 98 | 0.773 |
| F | 53 | 93.9245 | 9.32088 | ||||
| Average RNFL(µm) left | M | 47 | 91.9787 | 11.05813 | -0.428 | 98 | 0.67 |
| F | 53 | 93.1887 | 16.33902 | ||||
| Superior quadrant right | M | 47 | 120.128 | 14.66824 | 0.221 | 98 | 0.826 |
| F | 53 | 119.283 | 22.26534 | ||||
| Superior quadrant left | M | 47 | 122.234 | 19.32695 | -0.327 | 98 | 0.744 |
| F | 53 | 123.547 | 20.66357 | ||||
| Inferior quadrant right | M | 47 | 124.83 | 19.72123 | 0.502 | 98 | 0.617 |
| F | 53 | 122.943 | 17.88254 | ||||
| Inferior quadrant left | M | 47 | 120.723 | 24.13252 | 0.008 | 98 | 0.993 |
| F | 53 | 120.679 | 27.85455 | ||||
| Nasal quadrant right | M | 47 | 71.4894 | 11.25374 | 0.385 | 98 | 0.701 |
| F | 53 | 70.4717 | 14.66892 | ||||
| Nasal quadrant left | M | 47 | 66.4681 | 13.00644 | -0.626 | 98 | 0.533 |
| F | 53 | 68.7925 | 22.32139 | ||||
| Temporal quadrant right | M | 47 | 61.8936 | 10.02873 | -0.689 | 98 | 0.492 |
| F | 53 | 63.4717 | 12.53549 | ||||
| Temporal quadrant left | M | 47 | 61.0638 | 10.39315 | -0.842 | 98 | 0.402 |
| F | 53 | 63.283 | 15.17637 |
Table 2: Variation in the RNFL thickness between males (M) and females (F) in normal children <15 years measured by Spectral Domain OCT using Independent ‘t’ test.
Table 3 shows the inter-ocular variations in RNFL thickness in normal children measured by CIRRUS Spectral Domain OCT. The mean global RNFL thickness was 94.17 ± 8.96 SD in right eye and 92.62 ± 14.05 SD in left eye. The mean difference in global RNFL thickness between the two eyes was 1.55 with p value 0.35 which is not statistically significant (Table 4).
| Variable | Right Eye (RE) | Left Eye (LE) | RE vs. LE | ||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean Diff | t | p | |
| RNFL-FC | 94.17 ± 8.96 | 92.62 ± 14.05 | 1.55 | 0.93 | 0.35 |
| RNFL-S | 119.68 ± 18.98 | 122.93 ± 19.95 | -3.25 | -1.18 | 0.23 |
| RNFL-I | 123.83 ± 18.69 | 120.70 ± 26.44 | 3.13 | 0.98 | 0.33 |
| RNFL-N | 70.95 ± 13.11 | 67.7 ± 18.48 | 3.25 | 1.43 | 0.15 |
| RNFL-T | 62.73 ± 11.39 | 62.24 ± 13.13 | 0.49 | 0.28 | 0.77 |
Table 3: Comparison between the global RNFL and RNFL in each quadrant between two eyes.
| Parameters being correlated | N | Correlation (r) | p value |
|---|---|---|---|
| Age and average RNFL (µm) right eye | 100 | 0.0168 | 0.868 |
| Age and superior quadrant right eye | 100 | -0.0687 | 0.5 |
| Age and inferior quadrant right eye | 100 | 0.0749 | 0.457 |
| Age and nasal quadrant right eye | 100 | 0.071 | 0.48 |
| Age and temporal quadrant right eye | 100 | -0.0112 | 0.91 |
| Age and average RNFL (µm) Left eye | 100 | 0.0092 | 0.927 |
| Age and superior quadrant Left eye | 100 | -0.0424 | 0.678 |
| Age and inferior quadrant Left eye | 100 | 0.1198 | 0.23 |
| Age and nasal quadrant Left eye | 100 | -0.0465 | 0.64 |
| Age and temporal quadrant Left eye | 100 | 0.07 | 0.488 |
Table 4: Correlation of Age with RNFL thickness (Pearson’s correlation).
RNFL thickness varied minimally with age and gender whereas a moderate positive correlation was noted between the RNFL thickness in the inferior quadrant and refractive error of the right eye with a significant p-value of 0.023 and a moderate negative correlation was noted between the RNFL thickness in the temporal quadrant and refractive error of the right eye with a significant p value of 0.006 as shown in Table 5.
| Parameters being correlated | N | Correlation(r) | p-value |
|---|---|---|---|
| Refractive error (Spherical equivalent) and average RNFL(µm) of the right eye | 100 | 0.154 | 0.125 |
| Refractive error (Spherical equivalent) and Superior quadrant of the right eye | 100 | 0.143 | 0.155 |
| Refractive error (Spherical equivalent) and Inferior Quadrant of the right eye | 100 | 0.227 | 0.023 |
| Refractive error (Spherical equivalent) and Nasal quadrant of the right eye | 100 | 0.138 | 0.17 |
| Refractive error (Spherical equivalent) and Temporal quadrant of the right eye | 100 | -0.271 | 0.006 |
| Refractive error (Spherical equivalent) and average RNFL(µm) of the left eye | 100 | 0.079 | 0.437 |
| Refractive error (Spherical equivalent) and Superior quadrant of the left eye | 100 | 0.043 | 0.671 |
| Refractive error (Spherical equivalent) and Inferior quadrant of the left eye | 100 | 0.181 | 0.072 |
| Refractive error (Spherical equivalent) and Nasal quadrant of the left eye | 100 | 0.108 | 0.286 |
| Refractive error (Spherical equivalent) and Temporal quadrant of the left eye | 100 | -0.132 | 0.19 |
Table 5: RNFL thickness with refractive error (SE) (Pearsons correlation).
The mean Macular thickness was 269.56 μm. The mean central foveal thickness was 234.325 μm and the mean macular volume was 9.697 μm. Table 6 shows the inter-ocular variations in Macular thickness in normal children measured by CIRRUS Spectral Domain OCT. The mean Central foveal thickness, Macular thickness and macular volume was 235.02 ± 24.14 SD, 271.11 ± 14.07 SD and 9.749 ± 0.49 SD in right eye and 233.63 ± 24 SD, 268.01 ± 23.66 SD and 9.646 ± 0.84 SD respectively in left eye. The mean difference in central foveal thickness between the two eyes was 1.39 with p value 0.68 which is not statistically significant. The Macular thickness varied minimally with refractive error, gender and age as seen in Tables 7-9 respectively.
| Variable | Right Eye | Left Eye | RE vs. LE | ||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean Diff | t | p | |
| CFT | 235.02 ± 24.14 | 233.63 ± 24 | 1.39 | 0.41 | 0.68 |
| MT | 271.11 ± 14.07 | 268.01 ± 23.66 | 3.1 | 1.13 | 0.26 |
| MV | 9.749 ± 0.49 | 9.646 ± 0.84 | 0.103 | 1.05 | 0.29 |
Table 6: Comparison of the various macular thickness parameters between right and left eye.
| Parameters being correlated | N | Correlation(r) | p-value |
|---|---|---|---|
| Refractive error(Spherical equivalent) and GCC thickness of the right eye | 100 | -0.004 | 0.972 |
| Refractive error(Spherical equivalent) and GCC thickness of the left eye | 100 | -0.024 | 0.813 |
Table 7: Correlation of macular thickness parameters with refractive error
| Gender | N | Mean | Std. Deviation | t | df | p-value | |
|---|---|---|---|---|---|---|---|
| Central foveal thickness of right eye | M | 47 | 236.7234 | 23.98705 | 0.663 | 98 | 0.509 |
| F | 53 | 233.5094 | 24.40494 | ||||
| Central foveal thickness of left eye | M | 47 | 235.8511 | 25.72343 | 0.87 | 98 | 0.386 |
| F | 53 | 231.6604 | 22.42933 | ||||
| Macular thickness of right eye | M | 47 | 272.9149 | 14.14648 | 1.21 | 98 | 0.229 |
| F | 53 | 269.5094 | 13.94718 | ||||
| Macular thickness of left eye | M | 47 | 271.8511 | 18.45438 | 1.539 | 98 | 0.127 |
| F | 53 | 264.6038 | 27.19328 | ||||
| Macular volume of right eye | M | 47 | 9.8511 | 0.46526 | 1.469 | 97.2 | 0.145 |
| F | 53 | 9.6981 | 0.57462 | ||||
| Macular volume of left eye | M | 47 | 9.7021 | 0.71975 | 0.739 | 98 | 0.462 |
| F | 53 | 9.566 | 1.06535 |
Table 8: Correlation of macular thickness parameters with gender.
| Variable | Right Eye | Left Eye | RE vs. LE | ||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean | t | p | |
| GCC | 78.24 ± 14.36 | 78.6 ± 12.43 | -0.36 | -0.19 | 0.84 |
Table 9: Correlation of macular thickness parameters with age.
The mean GCC thickness was 78.42 μm. Table 10 shows the inter-ocular variations in Ganglion cell thickness in normal children measured by CIRRUS Spectral Domain OCT. The mean GCC thickness was 78.24 ± 14.36 SD in right eye and 78.6 ± 12.43 SD in left eye. The mean difference in GCC thickness between the two eyes was -0.36 with p value 0.84 which is not statistically significant. Tables 11-13 show there is no statistically significant correlation between GCC thickness with age, gender and refractive error.
| Parameters being correlated | N | Correlation(r) | p-value |
|---|---|---|---|
| Age and central foveal thickness of Right eye | 100 | 0.022 | 0.82 |
| Age and macular thickness of right eye | 100 | 0.0562 | 0.57 |
| Age and macular volume of right eye | 100 | 0.0697 | 0.49 |
| Age and central foveal thickness of left eye | 100 | 0.0677 | 0.5 |
| Age and macular thickness of Left eye | 100 | -0.0178 | 0.86 |
| Age and macular volume of left eye | 100 | -0.0258 | 0.804 |
Table 10: Comparison of the ganglion cell thickness parameters between right and left eyes.
| S. No | Parameters being correlated | N | Correlation(r) | p-value |
|---|---|---|---|---|
| 1 | Age and GCC thickness right | 100 | -0.009 | 0.92 |
| 2 | Age and GCC thickness left | 100 | 0.1258 | 0.21 |
Table 11: Correlation of GCC thickness with age.
| Gender | N | Mean | Std. Deviation | t | df | p-value | |
|---|---|---|---|---|---|---|---|
| GCC thickness-Right eye | M | 47 | 77.2766 | 15.21398 | -0.63 | 98 | 0.53 |
| F | 53 | 79.0943 | 13.6625 | ||||
| GCC thickness-Left eye | M | 47 | 77.2979 | 14.74702 | -0.964 | 79.2 | 0.338 |
| F | 53 | 79.7547 | 9.94776 |
Table 12: Correlation of GCC thickness with gender.
| Parameters being correlated | N | Correlation(r) | p-value |
|---|---|---|---|
| Refractive error (Spherical equivalent) and GCC thickness of the right eye | 100 | -0.004 | 0.972 |
| Refractive error (Spherical equivalent) and GCC thickness of the left eye | 100 | -0.024 | 0.813 |
Table 13: Correlation of GCC thickness with refractive error.
Discussion
The study titled "Peripapillary Retinal Nerve Fibre Layer Thickness, Ganglion Cell Thickness and Foveal thickness in school going children as measured by spectral domain Optical Coherence Tomography" was done in the Department of Ophthalmology, Father Muller Medical College; Mangalore. 100 children were included in the study which included 47 males and 53 females.
OCT has become widely used tool in clinical and scientific ophthalmology. Its uses in diagnosis of diseases are not restricted only to ophthalmology. Beside its use in identifying macular pathology and glaucoma, in recent year its application to diagnose various other ocular conditions has widely been expanded such as multiple sclerosis, optic nerve gliomas, pseudotumor cerebri, optic neuritis and papilloedema [5-8].
Normative data are provided automatically by OCT but the data base only includes individuals 18 years and above limiting its use in children. The application of OCT in children has been documented in several studies [9,10-12]. However, no normative data base exist which would serve as a benchmark for reference and glaucoma scanning [13].
Average RNFL thickness
The neuroretinal rim in normal eyes shows characteristic double hump configuration, which is usually the thickest in the inferior rim, followed by superior, nasal and the thinnest in the temporal rim, this is known as the Inferior Superior Nasal Temporal ISNT RULE. Several studies have confirmed this finding [14-16].
The average RNFL thickness in this study was 93.37 μm. When compared to other studies in the past, the average RNFL of our study was lower to those studies done previously. The mean RNFL in the females were slightly higher than the male with p=0.67 and was not statistically significant. The mean RNFL in RE was 94.17 μm and in the left eye was 92.62 μm with p=0.35. In a large study conducted by Huynh et al. [17] the average RNFL thickness was 103.7 ± 11.4 μm. The average RNFL in our study was lower to those of salchow et al. [18], Qian et al. [19], Dairi et al. [10], Leung et al. [4], and Ahn et al. [20].
Bourne et al. [21] compared the OCT 2000 with the Stratus OCT and found that the former model consistently yielded a higher RNFL thickness value. In comparison to the study conducted by Elai et al. [22], Barrio-Barrio et al. [23], Al-Haddad et al. [24] using Cirrus OCT yielded results that were consistent with our finding.
The RNFL thickness varies significantly among types of OCT used and therefore direct comparison of RNFL thickness measurement among OCT instrument like Stratus and Cirrus may be misleading [13].
The distribution of RNFL thickness (thickest inferiorly and superiorly and thinner nasally and temporally) is in agreement with the normal distribution of RNFL. These variations are the result of the large number of nerve fibres converging to the optic nerve head from the superior and inferior arcuate bundles, relative to the number of fibres converging from the papillomacular bundles and nasal retina.
Studies vary as to whether the RNFL was thicker temporally or nasally or whether it was thicker superiorly or inferiorly. In our study it was seen that superior RNFL was thinner compared to the inferior RNFL, with increasing age more thinning was seen in the superior RNFL compared to inferior RNFL, thinning was also seen in temporal and nasal RNFL; however these changes were not statistically significant.
RNFL thickness with age
Large number of studies has shown that RNFL thickness decreases as age increases [25-27]. It has been confirmed by several studies that the number of ganglion cells in human retina decreases with age which results in thinning of the RNFL. This has been confirmed by several investigation using OCT [1,28]. It has been estimated that normal individual loses ganglion cells at a rate of 4909 per year [29].
Bundez et al. [27] found that RNFL was thinner in older people with decline of approximately 2 microns per decade. Qian et al. [19] and Salchow et al. [18] reported that RNFL thickness tends to increase with age in a population younger than 18 years.
Alamouti et al. [28] studied 100 individual to establish changes in RNFL thickness with age in their study. They found highly significant correlation of both the retinal and the RNFL thickness with age. In these studies the retinal thickness decreased by 0.53 μm per year. About 80% of the changes in retinal thickness over time are caused by shrinkage of the RNFL. Poinooswamy et al. [30] examined 150 healthy volunteers of different ages using scanning laser polarimetry. They found a progressive reduction of the RNFL thickness with increasing age. The data presented in their study indicate a significant reduction of the RNFL thickness of 0.38 μm/year.
In this present study the mean age of the 100 participants were 12.07 ± 2.46 SD years (7-15 years). We analysed that there was mean global increase in the RNFL, as well as increase in RNFL in inferior, nasal and temporal quadrant with increasing age. The decrease in RNFL was more in superior quadrant compare to inferior quadrant, thinning was also seen in temporal quadrant as well as in nasal quadrant, but these changes were statistically insignificant.
In study conducted by Parikh et al, it was seen that RNFL tends to decrease with age. Average RNFL and RNFL by quadrant decreases especially after 50 years of age, thinning of the RNFL is not uniform in all with maximum loss in the superior quadrant in comparison to inferior quadrant which is more resistant to loss. This finding was consistent in our studies as well [31].
RNFL thickness with refraction
The effect of refractive error has been widely debated. Many studies have demonstrated positive correlation with spherical equivalent [10,17,19,31].
Huynh et al. [17] studies on 1765 children less than 6 years reported significant trend for thicker RNFL with more positive refraction, however the changes were small. Qian et al. [19] reported a positive correlation of the average RNFL thickness with refractive error in healthy children. Merugacz et al. [32] compared RNFL thickness between 30 myopic and 15 controlled participants without myopia and reported no significant difference between the two groups. Vernon et al. [33] conducted similar study on 31 highly myopic eye of caucasian origin and observed no statistically significant correlation between the RNFL and spherical equivalent. Rao et al. [13] found that axial length and refractive status accounted for only 10% of the variation in RNFL thickness. In our study, a poor positive correlation was established between refractive error and global RNFL thickness with a p value of 0.125 which was statistically insignificant.
Macular thickness
The central macular measurements obtained from the different OCT models in adults ranged from 257.6 to 277.1 μm. We found a lower central macular thickness with a mean of 234.325 μm than that observed in adults using the same OCT device [34]. Additionally, it was thinner than the other paediatric SD-OCT studies results, ranging from 253.8 μm to 271.2 μm [35-37].
There were different opinions on the relationship between macular thickness and age in adults. Some authors reported that the macular thickness was independent from age, while others [38] determined an age-related decrease in macular thickness which was compatible with the histological human retina studies presenting a decrease in the density of photoreceptors, ganglion cells, and retinal pigment epithelial cells with age [38,39]. In children, previous studies reported a significant increase in foveal thickness with age, specially beyond five years [35,37]. Our results were consistent with these observations.
Both adult and paediatric OCT studies have consistently documented differences between the gender in macular thickness regardless of age, with men/boys having thicker fovea than women/girls. The difference in macular thickness was found mainly in the fovea, inner macula, and outer temporal quadrant.35,36,39 Our findings are consistent with these observations.
Ganglion cell thickness
Jeanjean et al. [5] reported a significant negative correlation between average and sectorial GC-IPL thicknesses with AL in a study of normal Turkish children from 3 to 17 years old.
Previous studies in adults have attributed the thinning of average and segmental GC-IPL thickness values to stretching and consequent thinning of the retina [40,41]. Similar findings in children have also been reported by Lamirel et al. [6]. However, Lamirel et al. [6] had studied the GCC as a sum of RNFL and GC-IPL. The ganglion cells have the maximum density at the macula and it is suggested that study of this layer at the macula could be an early predictor of glaucoma and other optic nerve disease.
Pawar et al. [42] examined 139 children aged 3 to 18 years using SD-OCT and reported that the average GC-IPL thickness was 82.59 ± 6.29 μm, superior 83.68 μm, and inferior 81.64 μm. In our study, average GCC thickness was 78.42 μm. Avery et al. [7] evaluated the diagnostic abilities of SS-OCT and SD-OCT- obtained macular GC-IPL measurements in glaucoma patients and healthy adults aged 38 to 83, and found that the average GCIPL thickness in healthy eyes was 70.5 ± 5.5 μm using SS- OCT and 82.1 ± 6.6 μm using SD-OCT.
In our study, we additionally investigated the correlations between average GCC thickness and age, gender and SE. In our study there was a poor negative correlation established between GCC thickness and Spherical Equivalent. The mean GCC thickness was more in females than males regardless of age. We found that the average GCC thickness was not significantly associated with age. Contrastingly, Totan et al. found that average GC IPL was related to age (β, 0.226; p=0.009) and AL (β, -1.537; p<0.001), not to SE, rim area or disc area, while Goh et al. [42] determined that average GCIPL was correlated with AL (β, -2.056; p<0.001) though not with age.
Conclusion
Using Cirrus OCT, normative RNFL in healthy children between 7-15 years was established in our demographical set up. The retinal nerve fibre followed a normal distribution. RNFL, macular thickness and ganglion cell thickness varied minimally with age, gender, and refractive error. RNFL thinning was associated with less positive refraction.
The normative data from this study could serve as reference for further studies on paediatric glaucoma or other optic nerve head pathologies using nerve imaging modalities.
Limitations of the study
• All the subjects included in our study population were hospital based and hence do not reflect the general population.
• All children were between the age group of 7-15 years and therefore the result cannot be applied to the younger or older children accurately.
• The study is also limited by exclusion of high refractive error and limited number of subjects.
References
- Varma R, Bazzaz S, Lai M. Optical tomography-measured retinal nerve fibre layer thickness in normal Latinos. Invest Ophthalmol Vis Sci. 2003;44:3369-73.
- Chen TC, Zeng A, Sun W, et al. Spectral domain optical coherence tomography and glaucoma. Int Ophthalmol Clin. 2008;48:29–45.
- Hirasawa H, Tomidokoro A, Araie M et al. Peripapillary retinal nerve fiber layer thickness determined by spectral-domain optical coherence tomography in ophthalmologically normal eyes. Arch Ophthalmol. 2010;128:1420–6
- Leung CK, Cheung CY, Weinreb RN et al. Retinal nerve fibre layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116:1257–63.
- Jeanjean L, Castelnovo G, Carlander B, et al. Retinal atrophy using optical coherence tomography (OCT) in 15 patients with multiple sclerosis and comparison with healthy subjects. Rev Neurol (Paris). 2008;164:927-34.
- Lamirel C, Newman N, Biousse V. The use of optical coherence tomography in neurology. Rev Neurol Dis. 2009;6:E105-20.
- Avery RA, Liu GT, Fisher MJ, et al. Phillips. Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151:542-9.
- Kemenyova P, Turcani P, Sutovsky S, Waczulikova I. Optical coherence tomography and its use in optical neuritis and multiple sclerosis. Bratisl Lek Listy. 2014;115:723-9.
- Mrugacz M, Bakunowicz-Lazarczyk A. Optical coherence tomography measurement of the retinal nerve fiber layer in normal and juvenile glaucomatous eyes. Ophthalmologica. 2005;219:80-5.
- El-Dairi MA, Asrani SG, Enyedi LB, et al. Optical coherence tomography in the eyes of normal children. Arch Ophthalmol 2009; 127:50-8.
- Hess DB, Asrani SG, Bhide MG, et al. Macular and retinal nerve fiber layer analysis of normal and glaucomatous eyes in children using optical coherence tomography. Am J Ophthalmol 2005;139:509-17.
- Huynh SC, Wang XY, Rochtchina E, et al. Distribution of macular thickness by optical coherence tomography: Findings from a population based study of 6-year-old children. Invest Ophthalmol Vis Sci. 2006;47:2351-7.
- Rao A, Sahoo B, Kumar M, et al. Retinal nerve fiber layer thickness in children <18 years by spectral-domain optical coherence tomography. Semin Ophthalmol. 2013;28:97-102.
- Jonas JB, Gusek GC, Naumann GO. Optic disc cup and neuroretinal rim size configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988;29:1151-8.
- Mohammad Salih PA. Evaluation of peripapillary retinal nerve fiber layer thickness in myopic eyes by spectral domain optical coherence tomography. J Glaucoma. 2012;21:41-4.
- Dichtl A, Jonas JB, Naumann GO. Retinal nerve fiber layer thickness in human eyes. Graefes Arch Clin Exp Ophthalmol. 1999;237:474-9.
- Huynh SC, Wang XY, Rochtchina E, et al. Peripapillary Retinal nerve fiber layer thickness in a population of 6-year-old children. Ophthalmology. 2006;113:1583-92.
- Salchow DJ, Yuri S Oleynikov, Chiang MF, et al. Retinal nerve fiber layer thickness in normal children measured with optical coherence tomography. Ophthalmology. 2006;113:786-91.
- Qian J, Wang W, Zhang X, et al. Optical coherence tomography measurements of retinal nerve fiber layer thickness in Chinese children and teenagers. J Glaucoma. 2011;20:509-13
- Ahn HC, Son HW, Kim JS, et al. Quantitative analysis of retinal nerve fibre layer thickness of normal children and adolescents. Korean J Ophthalmol. 2005;19:195–200.
- Bourne RRA, Medeiros FA, Bowd C et al. Comparability of retinal nerve fibre layer thickness measurements of optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2005;46:1280-5.
- Elía N, Pueyo V, Altemir I, et al. Normal reference ranges of optical coherence tomography parameters in childhood. Br J Ophthalmol. 2012;96:665-70.
- Jesus Barrio-Barrio, Susana Noval, Marta Galdos, et al. Multicenter Spanish study of spectral domain optical coherence tomography in normal children. Acta Ophthalmol. 2013;91:56-63
- Al-Haddad C, Barikian A, Jaroudi M et al. Spectral domain optical coherence tomography in children: normative data and biometric correlations. BMC Ophthalmology. 2014,14:53.
- Knight OJ, Girkin CA, Budenz DL, et al. Effect of race, age, and axial length on optic nerve head parameters and retinal nerve fibre layer thickness measured by Cirrus HD-OCT. Arch Ophthalmol. 2012;130:312-8.
- Alasil T, Wang K, Keane PA. Analysis of normal retinal nerve fibre layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma. 2013;22:532-41.
- Budenz DL, Anderson DR, Varma R. Determinants of normal retinal nerve fibre layer thickness measured by Stratus OCT. Ophthalmology. 2007;114:1046-52.
- Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. Br J Ophthalmol. 2003;87:899-901.
- Mikelberg FS, Drance SM, Schulzer M et al. The normal human optic nerve. Axon count and axon diameter distribution. Ophthalmology. 1989;96:1325-8.
- Poinoosawmy D, Fontana L, Wu JX, et al. Variation of nerve fibre layer thickness measurements with age and ethnicity by scanning laser polarimetry. Br J Ophthalmol. 1997;81:350-4
- Tsai DC, Huang N, Hwu JJ, et al. Estimating retinal nerve fibre layer thickness in normal school children with spectral-domain optical coherence tomography. Jpn J Ophthalmol. 2012, 56:362-70.
- Mrugacz M, Bakunowicz-Lazarczyk A. Optical coherence tomography measurement of the retinal nerve fiber layer in normal and juvenile glaucomatous eyes. Ophthalmologica. 2005;219:80-5.
- Vernon SA, Rotchford AP, Negi A, et al. Peripapillary retinal nerve fibre layer thickness in highly myopic Caucasians as measured by Stratus optical coherence tomography. Br J Ophthalmol. 2008;92:1076-80.
- Liu T, Hu AY, Kaines A, et al. A pilot study of normative data for macular thickness and volume measurements using cirrus high-definition optical coherence tomography. Retina. 2011;31:1944-50.
- Barrio-Barrio J, Noval S, Galdos M, et al. Multicenter Spanish study of spectral-domain optical coherence tomography in normal children. Acta Ophthalmol. 2013;91:e56-63.
- Turk A, Ceylan OM, Arici C, et al. Evaluation of the nerve fibre layer and macula in the eyes of healthy children using spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;153:552-9.
- Yanni SE, Wang J, Cheng CS, et al. Normative reference ranges for the retinal nerve fiber layer, macula, and retinal layer thicknesses in children. Am J Ophthalmol. 2013;15: 354-360.
- Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M. Retinal photoreceptor density decreases with age. Ophthalmology. 1995;102:185-9.
- Ooto S, Hangai M, Sakamoto A, et al. Three-dimensional profile of macular retinal thickness in normal Japanese eyes. Invest Ophthalmol Vis Sci. 2010;51:465-73.
- Michael kaschke, Scott Meyer, Matt everett, et al. A Case Study in Medical Imaging Adoption optik and photonik. 2009;4:24-28.
- Sanchez-Galeana C, Bowd C, Blumenthal EZ, et al. Using optical imaging summary data to detect glaucoma. Ophthalmology. 2001;108:1812-8.
- Pawar N, Maheshwari D, Ravindran M, et al. Retinal Nerve Fiber Layer Thickness in Normal Indian Pediatric Population of North India Measured with Optical Coherence Tomography. Indian J Ophthalmol. 2014;62:412-8.