- Biomedical Research (2013) Volume 24, Issue 4
Perioperative High-Dose Amiodarone Elevates Nitric Oxide Levels in Patients Undergoing Coronary Artery Bypass Surgery.
Ayhan UYSAL1, Soner AZAK2, M.Cengiz COLAK3, Oktay BURMA1, I. Murat OZGULER1, Bilal USTUNDAG4, Mustafa Kemal BAYAR51Department of Cardiovascular Surgery, Firat University School of Medicine, 23119, Elazig, Turkey.
2Department of Cardiovascular Surgery, State Hospital, Giresun, 28100, Turkey.
3Department of Cardiovascular Surgery, Inonu University School of Medicine, 44100, Malatya, Turkey.
4Department of Biochemistry, Firat University School of Medicine, 23119, Elazig, Turkey.
5Department of Anaesthesiology, Firat University School of Medicine, 23119, Elazig, Turkey.
- Corresponding Author:
- Ayhan UYSAL
Department of Cardiovascular Surgery
Dicle University School of Medicine
21080 Diyarbakir, Turkey
Accepted Date: July 15 2013
Citation: Uysal A, Azak S, Colak MC, Burma O, Ozguler IM, Ustundag B, Bayar MK. Perioperative High-Dose Amiodarone Elevates Nitric Oxide Levels in Patients Undergoing Coronary Artery Bypass Surgery. Biomedical Research 2013; 24 (4): 486-492.
Abstract
The aim of the current study was to assess the effects of the Class III antiarrhythmic drug amiodarone on arterial blood Nitric oxide (NO) levels together with malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-px), and catalase (CAT) levels in patients undergoing coronary artery bypass surgery (CABG). Twenty patients undergoing CABG were included in the study. The patients were divided into control and amiodarone groups (n=10 in each group). The patients in group 1 did not receive any drugs. The patients in group II received 4X400 mg/day amiodarone on the day before surgery, 2X600 mg/day amiodarone on the day of surgery, and 2X400 mg/day amiodarone for the first consecutive four days after the surgery. NO, MDA, SOD, GSH-px, and CAT values were measured for biochemical evaluation of oxidative stress before the induction of anesthesia (TA), before CPB (TCPB), five minutes after the clamp was removed (Tc), after protamine (TP), and on postoperative days 1 (T1), 3 (T3), and 5 (T5). Hemodynamic changes of all patients were recorded at before the induction of anesthesia (TA), before CPB (TCPB), after protamine (TP), and on postoperative day 1 (T1). Amiodarone elevated NO levels at all times during the study period but did not cause changes in MDA, SOD, GSH-px, or CAT. In addition, amiodarone decreased mean pulmonary artery pressure, pulmonary capillary wedge pressure, and heart rate in these patients. No side effect due to drug was observed. Heart rate was found more decreased in amiodarone group at T1 and T2 stages when compared with controls (p<0.05). Perioperative high-dose amiodarone might be beneficial for patients who are pulmonary hypertensive and are undergoing CABG.
Keywords
Amiodarone, coronary bypass surgery, nitric oxide.
Introduction
Systemic oxidative stress on the human body is induced during cardiopulmonary bypass (CPB) and post-CPB, and neurological and cardiovascular side effects are observed in patients undergoing CPB. Thromboembolism, oxidative stress, and atrial fibrillation are some of the important side effects observed during or immediately after the surgery [1].
Amiodarone is one of the most effective Class III antiarrhythmic drugs frequently used in patients with AF [2]. It has also emerged as the leading antiarrhythmic therapy for the termination and prevention of ventricular arrhyth mia in different clinical settings because of its proven efficacy and safety [3]. Recently it has been found that a high dose of amiodarone in a short-term period reduces the incidence of postoperative AF and atrial flutter [4]. Additionally, dose depended vascular effects were reported in previous reports [5].
Although the effects of amiodarone on the post-ischemic heart and cardiomyocytes are well recognized, in vitro studies have shown that it also increases the amount of reactive oxygen species as measured by lipid peroxidation [6]. Albayrak et al. [7] also observed increased lipid peroxidation in vivo in rats given amiodarone for 30 days. These results show that at least some of the side effects of amiodarone might be due to increased oxidative stress. On the other hand, amiodarone appears to stimulate nitric oxide (NO) levels both in vitro and in vivo in studies [8, 9]. Taking into account possible beneficial effects of NO such as vasodilation and anti-aggregation of thrombocytes, new studies are needed to clarify the effects of amiodarone on oxidative stress and NO production in vivo in human subjects. Therefore, the aim of the current study was to assess the effects of perioperative high-dose amiodarone on blood NO, malondialdehyde, glutathione, catalase and superoxide dismutase levels in patients undergoing coronary bypass surgery.
Material and Methods
Patients
Twenty patients with coronary artery disease undergoing elective coronary bypass surgery were enrolled in this study. The study was approved by the Firat University Faculty of Medicine Ethical Committee on Human Research. Patients who had an active infection, thyroid disease, chronic obstructive disease, chronic renal failure, liver failure, or an operation history; or who were under chronic antiarrhythmic medication, had an emergency operation, reoperations, or additional surgical procedures; or who were over 80 years and had a heart rate less than 60/min and ejection fraction (EF) lower than 30% were not included. The patients were randomly divided into two groups (Group 1: control group, n=10; Group 2: amiodarone group, n=10). Randomization was stratified to assign 10 patients to the amiodarone group and 10 patients to the control group. Patients in Group 2 were given a total of 6 grams of amiodarone (Cordarone tablets, 200 mg Sanofi-Turkey) as follows: 400 mg 4 times on the day before operation, 600 mg 2 times on the day of operation, and 400 mg 2 times per day for the first four consecutive post-operative days. Each patient was given a Diazepam tablet (5mg) orally before admission to the operating room. A Swan–Ganz catheter (7F, Abbott Lab., USA) was placed via the right internal jugular vein, and a radial artery catheter and intravenous lines were placed. Anesthesia induction was provided with fentanyl (10 μg/kg, Fentanyl, Janssen), midazolam (0,1 mg/kg, Dormicum, Roche), and vecuronium (0,1 mg/kg, Norcuron, Organon Teknika). Anesthesia was maintained with sevoflurane (1- 2 %, concentration was adjusted according to clinical response) and additional fentanyl and vecuronium as necessary. Median sternotomy technique was used in all patients. The left internal thoracic artery and the vena safena magna were prepared for use as a graft.
CPB was completed with a roller pump (Stockert Instrumente, Germany), hollow fiber membrane oxygenator (Dideco D 708 Simplex III, Italy), and moderate hypothermia (28°C core temperature). 2000 ml Ringer’s solution was added to the prime solution. Anticoagulation was obtained by the administration of heparin (300 IU/kg, Liquemine, Roche), and activated clotting times (ACTs) were maintained at greater than 480 s with kaolincontaining tubes in all groups by the addition of heparin when necessary. Venous cannulation was done with a two-stage cannula from the right atrium. During total CPB, the aorta was clamped together with the pulmonary artery to prevent any antegrade flow to the lungs. Ventilation was stopped at this stage. Perfusion flow rate during CPB was maintained at 2.4 L/m2 per min. Myocardial preservation was achieved through antegrade administration of cold hyperkalemic blood (30 mEq/l K+) cardioplegic solution. This solution was repeated at 20 min intervals. Heparin antagonization was maintained with 1:1.3 protamine HCl (Protamine 1000, Roche). Nitroglycerin was not administered perioperatively to the patients.
Hemodynamic Measurements
Hemodynamic variables in all patients were evaluated for a total of four time periods: before induction of anesthesia (TA), before CPB (TCPB), after protamine infusion (TP), and on day 1 after surgery (T1). In addition, mean arterial pressure (MAP), heart rate (HR), central venous pressure (CVP), mean pulmonary artery pressure (MPAP), pulmonary capillary wedge pressure (PCWP), cardiac output (CO), and cardiac index (CI) values were assessed clinically. Cardiac output measurements using a Swan-Ganz catheter were performed by a cardiac output set (Edwards Lifesciences, USA) and cardiac output device (Spectramed Hemopro, USA).
Biochemical Analysis
Blood samples (8 ml) for biochemical assessments of oxidative stress were taken with the help of a catheter placed in the radial artery at seven time periods: before induction of anesthesia (TA), before CPB (TCPB), 5 min after cross clamp removal (Tc), after protamine infusion (TP), and on days 1 (T1), 3 (T3), and 5 (T5) after the surgery.
The determination of plasma malondialdehyde (MDA)
In vivo evaluation of free radicals in biological systems depends on the measurement of thiobarbituric acid reac tive products (TBARS). The end product (malondialdehyde, MDA) of lipid peroxidation was obtained by the method of Satoh [10] and Yagi [11] under aerobic conditions of pH 3.4. MDA levels were measured spectrophotometrically at a wavelength of 532 nm with the pinkcolored complex formed by incubation of plasma and thiobarbituric acid (TBA) in boiling water bath. The obtained values of MDA were expressed as nmol/ml.
The determination of plasma nitric oxide (NO)
Total nitrite (nitrate + nitrite) concentration was determined by the modified cadmium reaction method. To prevent non-specific reactions from the solutions of protein- rich nitrate plasma, the plasma had been previously deproteinized by Griess reaction as previous reports [12]. Then, under the pH 9.7 glycine buffer, copper (Cu)- coated cadmium granules were left by the supernatant samples at a 90-minute incubation; thus, nitrate reduction was achieved. The amount of nitrite produced was determined by the pink color formed by the reaction of sulfanilamide and the N-naftiletilendiamin sülfanilamid (NNDA) using a spectrophotometer at a wavelength of 545 nm. Results obtained using nitrite standards were calculated as mol/ml.
The determination of superoxide dismutase (SOD)
Superoxide dismutase activity in the plasma was measured according to the method of Sun et al. [13] by determining the reduction of nitro blue tetrazolium (NBT) by superoxide anion produced with xanthine and xanthine oxidase. One unit of SOD was defined as the amount of protein or hemoglobin that inhibits the rate of NBT reduction by 50%. Results were defined as units per gram hemoglobin (g Hb).
The determination of glutathione peroxidase (GSH-Px)
Glutathione peroxidase activity in the plasma was measured according to the method of Paglia and Valentine [14] by monitoring the oxidation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) at 340 nm. Enzyme units were defined as the number of micromoles of NADPH oxidized per minute. Results were defined as units per hemoglobin (g Hb).
The determination of catalase (CAT)
Catalase activity in the plasma was determined according to the method of Aebi [15] by monitoring the initial rate of disappearance of hydrogen peroxide (initial concentration = 10 mmol) at 240 nm in a spectrophotometer. Results were reported as constant rate per second per gram hemoglobin (K/g Hb).
Statistics
The primary endpoint of the present study was circulating NO levels. A sample size of 10 patients per group produced 80% power to detect a 20% change in circulating NO levels. The comparison of categorical variables between the groups was evaluated with chi-square test. In the comparison of demographic and clinical quantitative data, unpaired Student's t test, Mann-Whitney U test, and Wilcoxon test were used where appropriate. All p<0.05 values were considered significant. The data were expressed as mean ± standard deviation or frequencies. Statistical Program for Statistical Analysis Software System (SPSS) 15.0 was used in the analyses.
Results
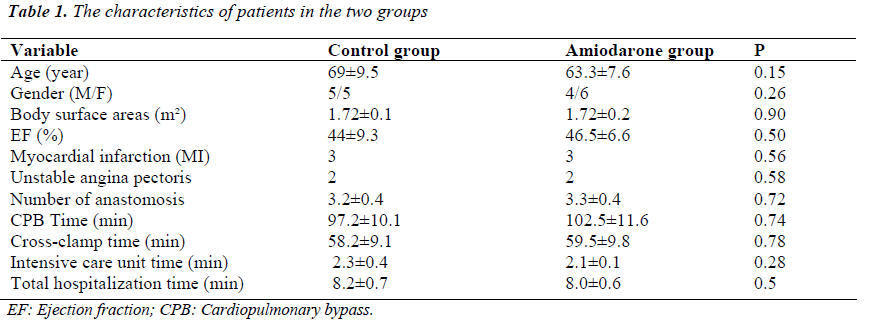
Patients in the control and amiodarone groups were compared with respect to age, gender, body surface areas (BSA), the values of pre-op ejection fraction, the numbers of anastomoses in the operation, CPB time, cross-clamp times, intensive care unit (ICU) time, total hospitalization time, and the other clinical features. With respect to these variables, there were no statistically significant differences between the groups (p>0.05) (Table 1).
Biochemical evaluation of the data
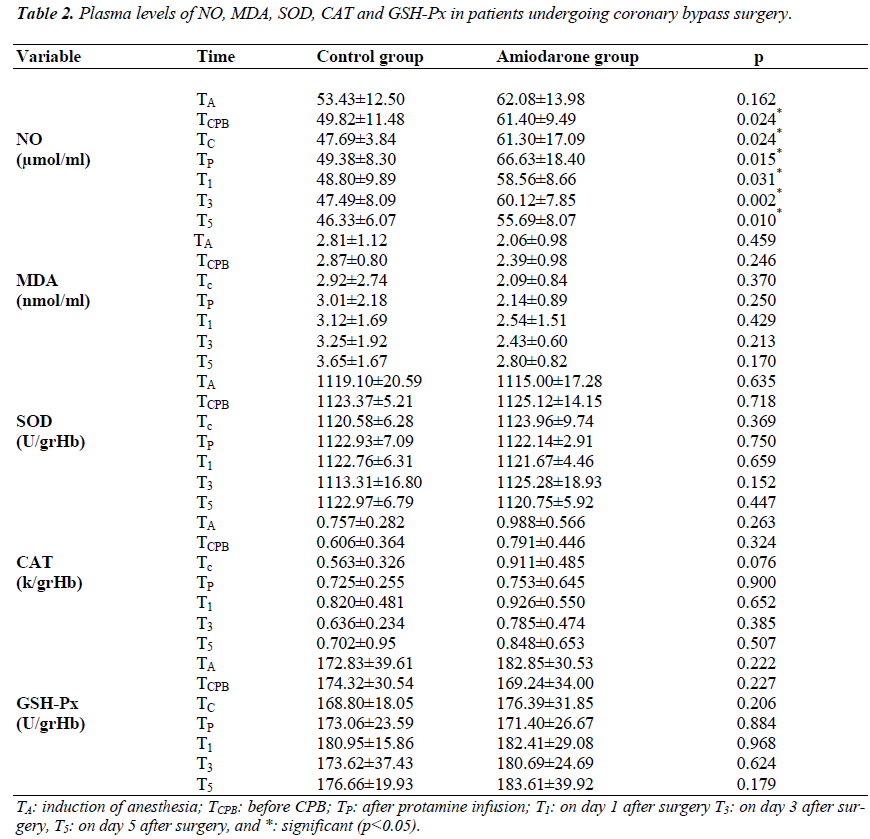
Plasma NO, MDA, SOD, CAT, and GSH-Px levels are presented in Table 2. NO levels were significantly higher in the amiodarone group at the time points during the study (p<0.05). Although MDA levels were slightly lower and CAT levels were slightly higher in the amiodarone group, there were no statistical differences between the groups (Table 2, p>0.05).
The evaluation of hemodynamic data
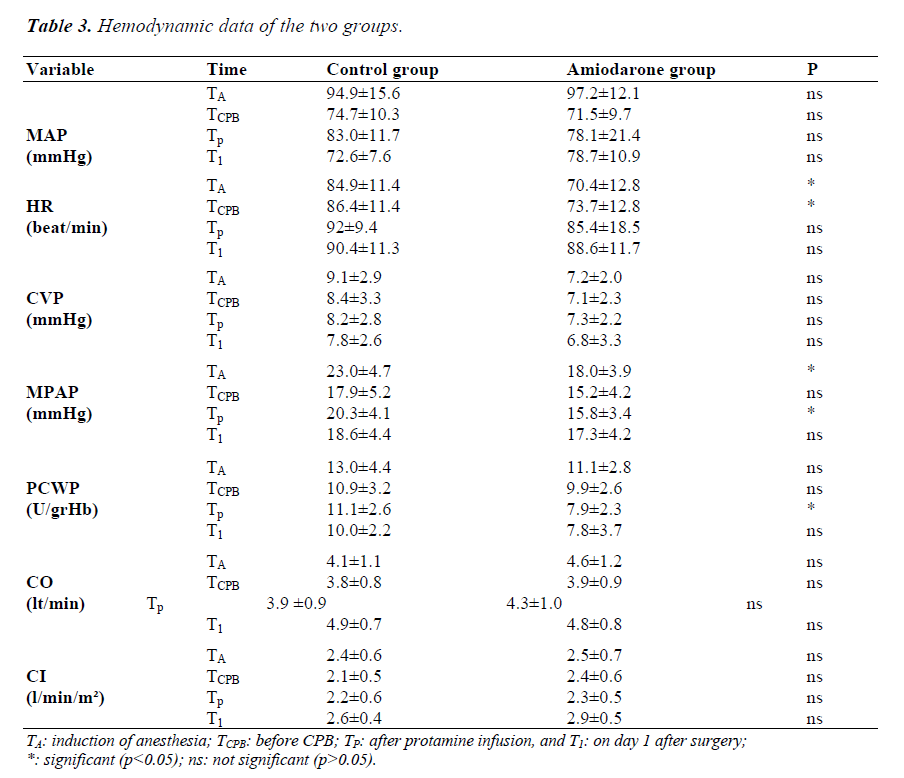
Table 3 summarizes the hemodynamic data for the time periods of the groups. Considering mean arterial pressure values, there was no significant difference between the two groups at any of the measurement times. Regarding heart rate, the values of TA and TCPB in the amiodarone group were decreased significantly compared to the control group (p<0.05). CVP values were lower in the amiodarone group than in the control group; however, these differences were not statistically significant (p>0.05). The values of TA and TC for MPAP were higher in the control group than in the amiodarone group (p<0.05). PCWP measurements were lower in the amiodarone group, but the decrease in TC period was significant (p<0.05). In relation to the measurements of CO and CI, no significant differences between the two groups were observed at any of the time points (p>0.05).
Discussion
Amiodarone is a class III anti-arrhythmic drug. In addition, class I (Na+ channel blockade), II (noncompetitive – adrenergic blockade) and IV (Ca++ channel blockade) properties, as well as a reserpine - like sympatholytic action, have been reported [16]. Ozbakis- Dengiz et al. [17] suggested that amiodarone dosedependently exerts a powerful anti-inflammatory activity. This effect of amiodarone may be due to the activation of nitric oxide resulting from its calcium channel antagonistic effects, to the inhibition of phospholipase A2 and/or to a reduction in neutrophil movement and activation, which may reduce free radical production and proteolytic enzyme release.
The present study clearly shows that high-dose amiodarone increases circulating NO levels in patients undergoing coronary bypass surgery and strengthens the data observed in vivo with laboratory animals. Endothelial NO synthase pathways are activated by amiodarone [18, 19]. An increase in plasma NO levels might have important consequences for both the intra- and postoperative periods. In fact, it has been shown that NO exerts important protective actions on the cardiovascular system [20, 21]. Beneficial effects of increased NO levels on the cardiovascular system include vasodilation and antithrombotic and anti-inflammatory properties [20]. Intraoperative use of NO as a vasodilator has been indicated for both cardiac and non-cardiac surgery [22]. Postoperatively, increased NO levels are associated with better outcomes for patients undergoing open-heart surgery, especially when pulmonary vasodilation is required both in congenital cardiac surgery and in adult patients with chronic pulmonary hypertension [23, 24, 25]. Therefore, increased levels of NO in the amiodarone group in the current study might be advantageous for patients undergoing cardiac surgery.
In the current study, lipid peroxidation (MDA levels) did not increase, suggesting that amiodarone does not contribute to the level of lipid peroxidation in patients undergoing cardiovascular bypass surgery as observed [26]. Hence, taken together with the data on NO levels and its potential beneficial effects, amiodarone might be beneficial for patients undergoing CPB.
Long-term amiodarone usage is associated with various side effects, including corneal microdeposits, optical neuritis, hypo- or hyperthyroidism, and irreversible pulmonary toxicity [27, 28]. In the current study, short-term high-dose amiodarone was preferred because Tuseth et al. [28] compared short-term high-doses of amiodarone (50 mg/hour vs 100 mg/hour doses over a 24 h period) and found that high doses result in lower heart rate and longer PR and QT intervals. The doses of amiodarone given in the current study are comparable to the high doses applied by Tuseth et al. [28]. Similarly, Alcalde et al. [4] gave oral amiodarone preoperatively at a dose of 1800 mg per day and observed postoperative improvements in the development of AF and atrial flutter. Additionally, Hofmann et al. [29] gave amiodarone intravenously at high doses (450 mg amiodarone i.v.) to patients with atrial fibrillation and concluded that this treatment was safe and effective in converting the patients to sinus rhythm. Taken together with the above data, the usage of amiodarone both pre- and postoperatively at high doses, as in the current study, seems to be beneficial. This notion is supported by the decreased MPAP, PCWP, and HR in the current study. None of the patients had bradycardia or atrial flutter disturbances in the postoperative period.
In conclusion, perioperative high-dose amiodarone increased circulating levels of NO but decreased MPAP, PCWP, and HR without elevating oxidative stress parameters. Therefore, amiodarone-associated increase in circulating NO might have important beneficial consequences in patients undergoing coronary bypass surgery.
Funding
The authors received no financial support for the research and/or authorship of this article.
Disclosures
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
References
- Marty JC, Bendhadra S, Amoureux S, et al. Oxidative stress is exacerbated in diabetic patients during cardiopulmonary bypass. Ann Cardiol Angeiol 2008; 57(3): 155-160.
- Kudenchuk PJ, Cobb LA, Copass MK, et al. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med 1999; 341(12): 871-878.
- Van Herendael H, Dorian P. Amiodarone for the treatment and prevention of ventricular fibrillation and ventricular tachycardia. Vasc Health Risk Manag. 2010; 6: 465-472.
- Alcalde RV, Guaragna JC, Bodanese LC, et al. High dose of amiodarone in a short-term period reduces the incidence of postoperative atrial fibrillation and atrial flutter. Arq Bras Cardiol 2006; 87(3): 236-240.
- Karahan O, Yavuz C, Demirtas S, Caliskan A, Atahan E. The investigation of the antiangiogenic potential of amiodarone HCl in the chick embryo chorioallantoic membrane model. Biomedical Research 2013; 24(1): 131-134.
- Golli-Bennour EE, Bouslimi A, Zouaoui O, Nouira S, Achour A, Bacha H. Cytotoxicity effects of amiodarone on cultured cells. Exp Toxicol Pathol 2012; 64(5): 425-430.
- Albayrak F, Bayir Y, Halici Z, et al. Preventive effect of amiodarone during acute period in isoproterenolinduced myocardial injury in Wistar rats. Cardiovasc Toxicol 2009; 9(4): 161-168.
- Guiraudou P, Pucheu SC, Gayraud R, et al. Involvement of nitric oxide in amiodarone- and dronedaroneinduced coronary vasodilation in guinea pig heart. Eur J Pharmacol 2004; 496(1-3): 119-127.
- Grossman M, Dobrev D, Kirch W. Amiodarone causes endothelium-dependent vasodilation in human hand veins in vivo. Clin Pharmacol Ther 1998 64(3): 302- 311.
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta 1978; 90: 37–43.
- Yagi K. Assay of blood plasma or serum for serum lipid perokside level and its clinical signifance. Methods in Enzymology. 1984; 105: 224–241.
- Yavuz C, Yazici S, Karahan O, et al. Serum nitric oxide level could be a predictive biomarker for detection of critical ischaemia duration. Biomarkers. 2013; 18(2): 116-120.
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; 34(3): 497-500.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967; 70(1): 158- 69.
- Aebi H. In Bergmeter HU(ed) Methods in Enzymatic Analysis, Weinheim, Verlag Chemie 1982;3: 273-282.
- Oliviera PF, Dias da Silva VJ, Salgado MCO, Fazan Jr R, Aguiar CA, Salgado HC. Acute effect of amiodarone on cardiovascular reflexes of normotensive and renal hypertensive rats. Braz J Med Biol Res 2005; 38: 967-976.
- Ozbakis-Dengiz G, Halici Z, Akpınar E, Cadirci E, Bilici D, Gursan N. Role of polymorphonuclear infiltration in the mechanism of anti-inflammatory effect of amiodarone. Pharm Rep 2007; 59: 538-544.
- Grossmann M, Dobrev D, Himmel HM, Kirch W. Local venous response to N-desethylamiodarone in humans. Clin Pharmacol Ther. 2000; 67(1): 22-31.
- Kishida S, Nakajima T, Ma J, et al. Amiodarone and NDesethylamiodarone Enhance Endothelial Nitric Oxide Production in Human Endothelial Cells. Int Heart J. 2006; 47: 85-93.
- Gkaliagkousi E, Ferro A. Nitric oxide signalling in the regulation of cardiovascular and platelet function. Front Biosci. 2011; 16: 1873-1897.
- Akar I, Rahman A, Colak MC, Üstündağ B, Özercan IH, Uysal A. Immunohistochemical evaluation of the effects of nebivolol on intimal hyperplasia following endothelial injury. Turk J Med Sci 2011; 41(1): 53-63.
- Kleen M, Zwissler B. Intra-operative use of inhaled vasodilators: are there indications? Curr Opin Anaesthesiol 2002; 15(1): 79-83.
- Murthy KS, Rao SG, Prakash KS, Robert C, Dhinakar S, Cherian KM. Role of inhaled nitric oxide as a selective pulmonary vasodilator in pediatric cardiac surgical practice. Indian J Pediatr 1999; 66(3): 357-361.
- Liu YL, Hu SS, Shen XD, et al. Indications of arterial switch operation for complex congenital heart defect with severe pulmonary hypertension and ventriculoarterial discordant connection. Zhonghua Yi Xue Za Zhi. 2006; 86(1): 23-25.
- Zhang XY, Yang ZJ. Perioperative nitric oxide inhalation therapy for open heart surgery patients with pulmonary hypertension. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007; 19(9): 546-548.
- Ribeiro SM, Campello AP, Nascimento AJ, Kluppel ML. Effect of amiodarone (AMD) on the antioxidant enzymes, lipid peroxidation and mitochondrial metabolism. Cell Biochem Funct. 1997; 15(3): 145-152.
- Sohns C, Zabel M. Current role of amiodarone in antiarrhythmic therapy. Herzschrittmacherther Elektrophysiol. 2010; 21(4): 239-243.
- Tuseth V, Jaatun HJ, Dickstein K. Amiodarone infuseion in the treatment of acute atrial fibrillation or flutter: high versus low dose treatment. Heart. 2005; 91(7): 964-965.
- Hofmann R, Steinwender C, Kammler J, Kypta A, Leisch F. Effects of a high dose intravenous bolus amiodarone in patients with atrial fibrillation and a rapid ventricular rate. Int J Cardiol 2006; 110(1): 27- 32.