- Biomedical Research (2015) Volume 26, Issue 4
Pentoxifylline inhibits angiotensin II-induced proliferation in rat vascular smooth muscle cells
Sang Youb Han1,2, Cy-Hyun Kim2,3, Yoon Mee Lee2, Dae Hee Kim2, Kum-Hyun Han1, and Dae Ryong Cha31Department of Internal Medicine, Goyang, Korea
2Clinical Research Center, Inje University Ilsan-Paik Hospital, Goyang, Korea
3Departments of Internal Medicine, Korea University Ansan Hospital, Ansan, Korea
Accepted Date: August 07 2015
Abstract
We tested the ability of the drug pentoxifylline to inhibit the activity of the vasoactive hormone angiotensin II in vascular smooth muscle cells. Cell proliferation, intracellular cAMP, and expression of cell cycle proteins were measured in cells isolated from male Sprague-Dawley rats. Angiotensin II significantly induced proliferation in VSMCs (p < 0.01). Pentoxifylline significantly blocked this induction in a dose-dependent manner (p < 0.05), and a dose of 0.5 mM was sufficient to completely reverse the effect of angiotensin II. Angiotensin II reduced production of cAMP from 34.62 ± 0.59 pmol/mg protein in untreated cells to 17.49 ± 3.30 pmol/mg protein (p < 0.05). cAMP production was restored to 40.68 ± 0.49 and 41.50 ± 1.78 pmol/mg protein in the presence of 1.0 and 2.0 mM pentoxifylline, respectively. In addition, the same treatments increased cAMP production in untreated cells to 54.82 ± 4.40 and 67.68 ± 4.29 pmol/mg protein, respectively (p < 0.05). Angiotensin II significantly upregulated (p < 0.05) cyclin D1 mRNA 2-fold, but not cyclin E, cyclin A, CDK2, CDK4, or P27. Pretreatment with pentoxifylline prevented this effect. Similarly, the drug blocked the ability of angiotensin II to increase the abundance cyclin D1 protein. Conclusion: Pentoxifylline attenuated angiotensin II-induced proliferation by stimulating cAMP production and partially regulating the cell cycle. However, studies are required to investigate the effects of the drug on the hypertensive vessel wall.
Keywords
Pentoxifylline, Angiotensin II, vascular smooth muscle, cell proliferation, cAMP
Introduction
The renin-angiotensin system is pathologic in vascular injury [1,2]. In this system, the vasoactive hormone angiotensin II constricts and enhances resistance in blood vessels to increase blood pressure [3]. In addition, the hormone stimulates DNA synthesis, cell division, and proliferation in vascular smooth muscle cells (VSMCs) [4], which maintain vascular integrity and tone. Under pathologic conditions, these cells also remodel arteries [5,6], especially when stimulated by mitogens such as angiotensin II, platelet-derived growth factor, and transforming growth factor-β1 [7]. While vascular injury is complex and attributable to many factors such as cell migration, growth, apoptosis, calcification, and inflammation [7,8], the activities of angiotensin II indicate that it is a major catalyst of hypertensive vascular damage due to tissue remodeling [9].
Pentoxifylline, a non-selective inhibitor of cyclic-3’,5’-phosphodiesterase, is used to treat peripheral vascular disease [10]. The drug inhibits cell proliferation, inflammation, and fibrosis [11,12] via the cAMP pathway [13]. Although cAMP is well known to modulate vascular proliferation, it is unclear whether pentoxifylline could block angiotensin II-induced proliferation in VSMCs, or whether its anti-proliferative activity is linked to cell cycle proteins. Therefore, we analyzed the effect of the drug on proliferation and the cell cycle in angiotensin IIstimulated cells.
Materials and Methods
Cell culture
VSMCs were isolated from the thoracic aorta of male Sprague-Dawley rats (weight, 180–200 g). Cells were grown to subconfluence at 37 °C in a humidified atmosphere of 95% air and 5% CO2 in Dulbecco’s modified Eagle’s media supplemented with 10% fetal bovine serum, 25 mM HEPES pH 7.4, 100 U/mL penicillin, and 100 μg/mL streptomycin. After 4–8 passages, cells were cultured for 24 h in 0.1% fetal bovine serum prior to experiments, in which cells were cultured in 5.5 mM normal glucose, pretreated for 30 min with pentoxifylline, and stimulated with 1 μM angiotensin II (Sigma-Aldrich, St. Louis, USA). Cells were harvested 24 h thereafter for analysis of mRNA and protein expression. Data were collected from triplicate experiments.
Proliferation assay
Cells were seeded in 96-well plates at 1 × 105 cells/well, and grown to confluence, at which point the medium was replaced by fresh serum-free medium. Cultures were then incubated at 37 °C for 4 h with 100 μL 0.5 mg/mL 3-(4, 5-di-methylthizol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT), washed with phosphate-buffered saline, lysed with 100 μL dimethyl sulfoxide, and shaken for 10 min. Finally, absorbance was measured at 570 nm using an automatic microplate reader to determine the amount of MTT reduced to formazan (Molecular Devices, Sunnyvale, CA)
cAMP assay
Media were removed from cultures, and cells were incubated for 20 min at room temperature in 500 μL cold 0.1 N HCl. Cells were then scraped and pelleted by centrifugation at 1000 ×g for 10 min. Extracts were acetylated, and cAMP content was measured by an enzyme immunoassay kit (Cayman Chemical Company, Ann Arbor, USA), following the manufacturer’s instructions. cAMP levels were normalized to protein concentrations, which were determined using Advanced Protein Assay Reagent (Bio-Rad, Hercules, USA).
Real time reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol and was reversetranscribed using a cDNA synthesis kit (Fermentas, Burlington, Canada) as previously described [14]. Gene expression was measured by real time RT-PCR using the SYBR Green Master mix and standard three-step cycles. Data were normalized to those of the housekeeping gene GAPDH. Primers were designed using Primer 3 software and had the following sequences: cyclin A1, sense 5’ CAGTACTTAAGGCGGCAAGG 3' and anti-sense 5’ TAAGCTGCTGCTGCTACCAA 3'; cyclin D1, sense 5’ AGGGGATTCAGGACGACTCT 3' and anti-sense 5’ GGGCAACCTTCCCAATAAAT 3'; cyclin E1, sense 5’ ATGTCCAAGTGGCCTACGTC 3' and anti-sense 5’ GCGAGGACACCATAAGGAAA 3'; P27, sense 5’ CAGAATCATAAGCCCCTGGA 3' and anti-sense 5’ TCTGACGAGTCAGGCATTTG 3'; cyclin-dependent kinase 4 (CDK4), sense 5’ GAAGACGACTGGCCTCGAGA 3' and anti-sense 5’ ACTGCGCTCCAGATTCCTCC 3'; CDK2, sense 5’ TGACCAACTCTTCCGGATCT 3' and anti-sense 5’ ATAACAAGCTCCGTCCGTCT 3'; and GAPDH, sense 5’ TGCACCACCAACTGCTTAGC 3' and anti-sense 5’ GGCATGGACTGTGGTCATGAG 3'.
Western blot
Cells were lysed in buffer containing 150 mM NaCl, 50 mM Tris-HCl, pH 8.0, 1% Triton X-100, and 1 mM phenylmethylsulfonylfluoride, and total protein concentrations in extracts were determined with Advanced Protein Assay Reagent (Bio-Rad, Hercules, USA). Samples containing 40 μg protein were separated on 6 or 10% SDS-polyacrylamide gels under denaturing conditions and blotted onto polyvinylidene difluoride (Immobilon-P, Millipore, USA) for 120 min at 250 mA. Blots were blocked for 1 h at room temperature with 0.15% Tween-20 and 5% non-fat milk in phosphatebuffered saline, and probed overnight at 4 °C with monoclonal antibodies against rat cyclin D (Cell Signaling, USA, 1:500), and β-actin (Santa-Cruz, USA, 1:3000). Membranes were then washed 4 times with Tween-20 in phosphate-buffered saline, and labeled for 30 min at room temperature with a 1:3000 dilution of horseradish peroxidase-conjugated secondary antibody. Blots were visualized by the ECL method (Amersham, Buckinghamshire, UK).
Statistical analysis
Data were analyzed in the statistical program SPSS 15.0 using non-parametric Mann-Whitney Room corporate law was used . p < 0.05 was considered statistically sig nificant.
Results
Anti-proliferative activity of pentoxifylline
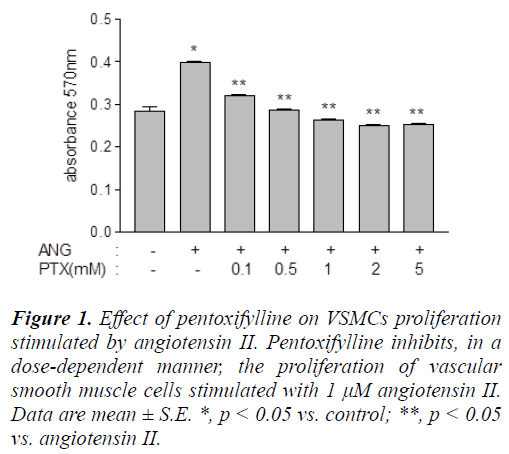
Angiotensin II significantly induced (p < 0.0001) proliferation in VSMCs, as measured by the amount of MTT reduced to formazan, the formation of which was quantified by absorbance at 570 nm (Figure 1). The absorbance at 570 nm was 0.29 ± 0.02 in untreated cells, and 0.40 ± 0.05 in hormone-stimulated cells. Pentoxifylline significantly blocked (p < 0.05) this induction in a dose-dependent manner, and a dose of 0.5 mM was sufficient to completely reverse the effect of angiotensin II, and reduce the absorbance to 0.29 ± 0.03 (Figure 1).
Figure 1: Effect of pentoxifylline on VSMCs proliferation stimulated by angiotensin II. Pentoxifylline inhibits, in a dose-dependent manner, the proliferation of vascular smooth muscle cells stimulated with 1 μM angiotensin II. Data are mean ± S.E. *, p < 0.05 vs. control; **, p < 0.05 vs. angiotensin II.
Effect of pentoxifylline on intracellular cAMP
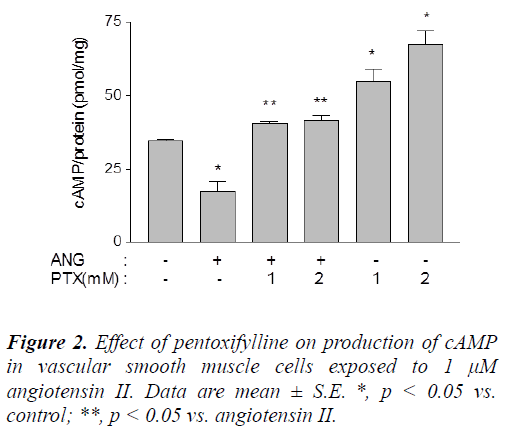
Angiotensin II reduced production of cAMP from 34.62 ± 0.59 pmol/mg protein in untreated cells to 17.49 ± 3.30 pmol/mg protein (p < 0.05). cAMP production was restored to 40.68 ± 0.49 and 41.50 ± 1.78 pmol/mg protein in the presence of 1.0 and 2.0 mM pentoxifylline, respectively. In addition, the same treatments increased cAMP production in untreated cells to 54.82 ± 4.40 and 67.68 ± 4.29 pmol/mg protein, respectively (p < 0.05) (Figure 2).
Cell cycle regulation by pentoxifylline
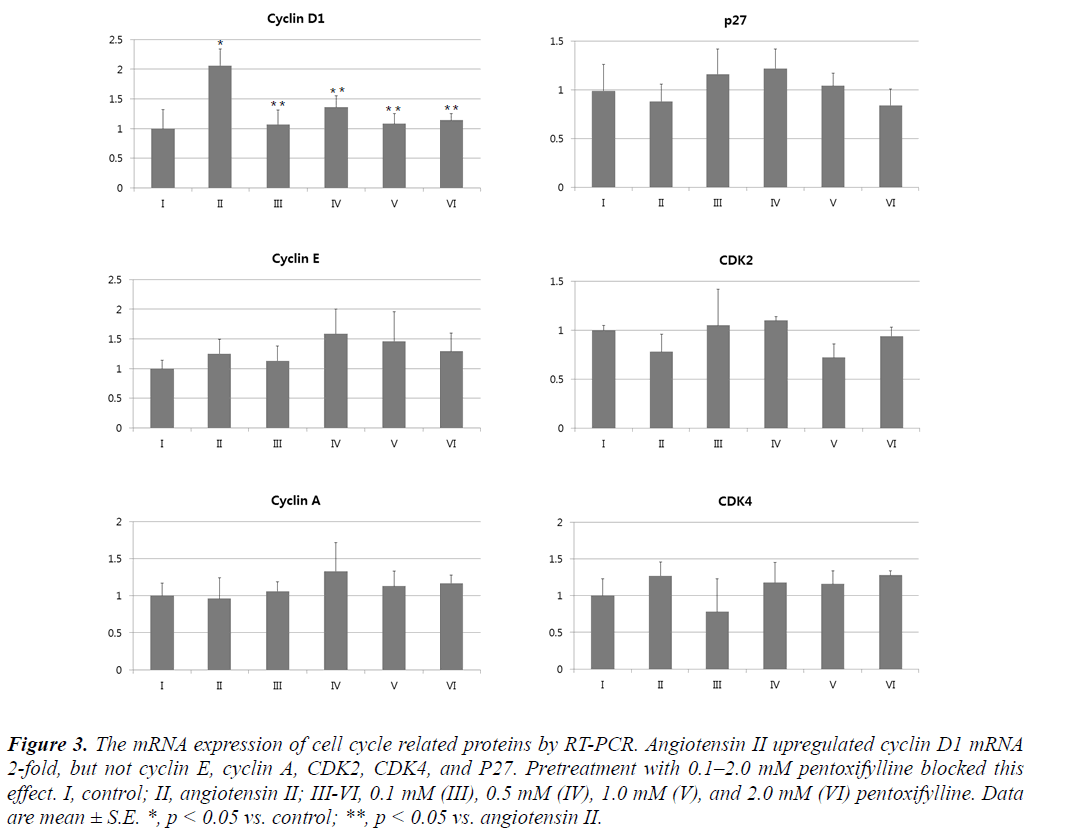
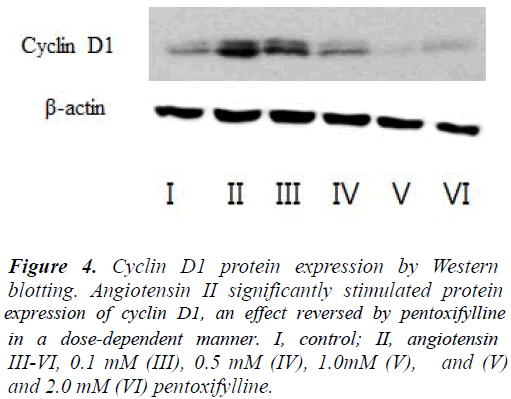
Angiotensin II significantly upregulated (p < 0.05) cyclin D1 mRNA 2-fold, but not cyclin E, cyclin A, CDK2, CDK4, or P27 (Figure 3). Pretreatment with pentoxifylline prevented this effect. Similarly, the drug blocked the ability of angiotensin II to increase the cyclin D1 protein (Figure 4).
Figure 3: The mRNA expression of cell cycle related proteins by RT-PCR. Angiotensin II upregulated cyclin D1 mRNA 2-fold, but not cyclin E, cyclin A, CDK2, CDK4, and P27. Pretreatment with 0.1–2.0 mM pentoxifylline blocked this effect. I, control; II, angiotensin II; III-VI, 0.1 mM (III), 0.5 mM (IV), 1.0 mM (V), and 2.0 mM (VI) pentoxifylline. Data are mean ± S.E. *, p < 0.05 vs. control; **, p < 0.05 vs. angiotensin II.
Figure 4: Cyclin D1 protein expression by Western blotting. Angiotensin II significantly stimulated protein expression of cyclin D1, an effect reversed by pentoxifylline in a dose-dependent manner. I, control; II, angiotensin III-VI, 0.1 mM (III), 0.5 mM (IV), 1.0mM (V), and (V) and 2.0 mM (VI) pentoxifylline.
Discussion
Accelerated proliferation in vascular smooth muscle cells is an important factor in the development of hypertension [15]. The vasoactive hormone angiotensin II elicits such acceleration via several mechanisms. For instance, the mitogenic factors platelet-derived growth factor and transforming growth factor-β1 enhance angiotensin II activity [16,17]. The hormone also induces production of mitochondrial reactive oxygen species, which are associated with hypertension and atherosclerosis [18]. Moreover, angiotensin II stimulates NADPH oxidase [19], which contributes to cell growth and inflammation in atherosclerosis [20]. Finally, the hormone inhibits phosphate and tensin homolog to promote cell proliferation and migration [21] and upregulates extracellular signal-regulated kinase in response to high amounts of sodium [22].
In contrast, intracellular cAMP maintains vascular smooth muscle cells in the quiescent state under physiological conditions and facilitates injury repair by inhibiting proliferation [13,23,24]. Thus, a specific inhibitor of PDE 3 and 4 induces vascular relaxation and prevents proliferation by increasing cAMP [25,26]. Accordingly, we hypothesized that pentoxifylline, a drug that also increases intracellular cAMP, may block the ability of angiotensin II to stimulate vascular cell proliferation. Indeed, our data demonstrate that pentoxifylline attenuates proliferation in hormone-stimulated vascular smooth muscle cells via cAMP production. This finding is consistent with previous results [27,28].
In particular, cAMP blocks cell cycle progression by inhibiting cyclin D, and c-myc [23,29]. Cyclin D1 regulates progression from the G1 to S phase [30,31]. In contrast, angiotensin II stimulates cyclin D1 and cyclin E and inhibits p27 [32,33], ultimately inducing proliferation. On the other hand, studies have demonstrated that pentoxifylline prevents platelet-derived growth factor from stimulating cyclin D1 expression in mesangial cells and airway smooth muscle cells [11,34]. In line with these data, we found pentoxifylline to attenuate expression of cyclin D1 after exposure to angiotensin II, but not other cell cycle proteins.
In summary, pentoxifylline attenuates proliferation in angiotensin II-stimulated vascular smooth muscle cells by increasing cAMP and partially regulating the cell cycle. Similarly, the drug has been reported to inhibit proliferation in other cells, including astrocytes, fibroblasts, hepatic stellate cells, and mesangial cells [35-38]. In vivo, the drug inhibits vascular proliferation in models of carotid arterial anastomosis model and angioplasty [39,40]. However, further studies are required to define pentoxifylline activity in the hypertensive vessel wall. Nevertheless, the data imply that administration of pentoxifylline may be an alternative strategy to prevent vascular proliferation and constriction in pathological conditions.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
Acknowledgement
This work was supported by the Postdoctoral Research Program of Inje University 2007.
References
- Schiffrin EL, Touyz RM. From bedside to bench tobedside: role of renin-angiotensin-aldosterone system in remodeling of resistance arteries in hypertension. Am J Physiol Heart CircPhysiol 2004; 287(2): H435- 446.
- Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. CircRes 2002; 90(11): 1205-2013.
- Cheng ZJ, Vapaatalo H,Mervaala E. Angiotensin II and vascular inflammation. Med SciMonit 2005; 11(6): RA194-205.
- Herbert KE, Mistry Y, Hastings R, Poolman T, Niklason L, Williams B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. CircRes 2008; 102(2): 201-208.
- Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res 2012; 95(2): 194-204.
- Savoia C, Burger D, Nishigaki N, MontezanoA, TouyzRM. Angiotensin II and the vascular phenotype in hypertension. Expert Rev Mol Med 2011; 30: 13:e11.
- Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 200; 38(3 Pt 2): 581-587.
- Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 2009; 24: 45-57.
- Hitomi H, KaifuK, Fujita Y, Sofue T, Nakano D, Moriwaki K, Hara T, Kiyomoto H, Kohno M, KoboriH, Nishiyama A. Angiotensin II shifts insulin signaling into vascular remodeling from glucose metabolism in vascular smooth muscle cells. Am J Hypertens 2011; 24(10): 1149-1155.
- Stevens JW, Simpson E, Harnan S, Squires H, Meng Y, Thomas S, Michaels J, Stansby G. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg2012; 99(12): 1630-1638.
- Lin SL, Chen RH, Chen YM, Chiang WC, Tsai TJ, Hsieh BS. Pentoxifylline inhibits platelet-derived growth factor-stimulated cyclin D1 expression in mesangial cells by blocking Akt membrane translocation. Mol. Pharmacol 2003; 64: 811-822.
- Chen YM, Ng YY, Lin SL, Chiang WC, Lan HY, Tsai TJ. Pentoxifylline suppresses renal tumour necrosis factor-a and ameliorates experimental crescenticglomerulonephritis in rats. Nephrol. Dial. Transplant 2004; 19: 1106-1115.
- Rossi F, Bertone C, Petricca S, Santiemma V. Adrenomedullin antagonizes angiotensin II-stimulated proliferation of human aortic smooth muscle cells. Peptides 2006; 27(11): 2935-2941
- Sun HK, Lee YM, Han KH, Kim HS, Ahn SH, Han SY.Phosphodiesteraseinhibitor improves renal tubulointerstitial hypoxia of the diabetic rat kidney. Korean J Intern Med. 2012; 27(2): 163-170.
- Mikhail N, Fukuda N, Tremblay J, Hamet P. Platelets, growth factors, and vascular smooth-muscle cells in hypertension and diabetes. J CardiovascPharmacol1993; 22 Suppl 6: S64-74.
- Satoh C, Fukuda N, Hu WY, Nakayama M, KishiokaH, Kanmatsuse K. Role of endogenous angiotensin II in the increased expression of growth factors in vascular smooth muscle cells from spontaneously hypertensive rats. J CardiovascPharmacol 2001; 37(1): 108-118.
- Bobik A. Hypertension, transforming growth factorbeta,angiotensin II and kidney disease. J Hypertens2004; 22(7): 1265-1267.
- Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal 2013; 19(10): 1085- 1094.
- Montezano AC, Touyz RM. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid Redox Signal 2014; 20(1): 164-182.
- Holterman CE, Thibodeau JF, Towaij C, Gutsol A, Montezano AC, Parks RJ, Cooper ME, Touyz RM, Kennedy CR. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J Am SocNephrol 2014; 25(4): 784-797
- Dong X1, Yu LG, Sun R, Cheng YN, Cao H, Yang KM, Dong YN, Wu Y, Guo XL. Inhibition of PTEN expression and activity by angiotensin II induces proliferation and migration of vascular smooth muscle cells. J Cell Biochem. 2013; 114(1): 174-182. .
- Liu G, Hitomi H, Rahman A, Nakano D, Mori H, Masaki T, Ma H, Iwamoto T, Kobori H, Nishiyama A. High sodium augments angiotensin II-induced vascular smooth muscle cell proliferation through the ERK 1/2- dependent pathway. Hypertens Res 2014; 37(1): 13-18.
- Wu Y, Bond M, Sala-Newby G, Newby A. Altered Sphasekinase-associated protein-2 levels are a major mediator of cyclic nucleotide-induced inhibition of vascular smooth muscle cell proliferation. Circ Res 2006; 98: 1141–1150.
- Indolfi C, Avvedimento EV, DiLorenzo E, Esposito G, Rapacciuolo A, Giuliano P, Grieco D, Cavuto L, Stingone AM, Ciullo I, Condorelli G, Chiariello M. Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat Med 1997; 3: 775–779.
- Pan X, Arauz E, Krzanowski JJ, Fitzpatrick DF, Polson JB. Synergistic interactions between selective pharmacological inhibitors of phosphodiesteraseisozyme families PDE III and PDE IV to attenuate proliferation of rat vascular smooth muscle cells.BiochemPharmacol 1994; 48(4): 827-835.
- Nishimura J. Topics on the na(+)/ca(2+) exchangerinvolvement of na(+)/ca(2+) exchanger in the vasodilator-induced vasorelaxation. J PharmacolSci2006; 102(1): 27-31.
- Chen YM, Wu KD, Tsai TJ, Hsieh BS.Pentoxifyllineinhibits PDGF-induced proliferation of and TGF-betastimulated collagen synthesis by vascular smooth muscle cells. J Mol Cell Cardiol 1999; 31(4): 773-783.
- Kip SN, Hunter LW, Ren Q, Harris PC, Somlo S, Torres VE, Sieck GC, Qian Q. [Ca2+]i reduction increases cellular proliferation and apoptosis in vascular smooth muscle cells: relevance to the ADPKD phenotype. Circ Res. 2005; 96(8): 873-880.
- L'Allemain G1, Lavoie JN, Rivard N, Baldin V, Pouyssegur J. Cyclin D1 expression is a major target of the cAMP-induced inhibition of cell cycle entry in fibroblasts. Oncogene. 1997; 14(16): 1981-1990.
- Sherr CJ. G1 phase progression: Cycling on cue. Cell 1994;79:551–555.
- Lavoie JN, Allemain GL, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J BiolChem 1996; 271: 20608–20616.
- Kim JE, Choi HC. Losartan Inhibits Vascular Smooth Muscle Cell Proliferation through Activation of AMPActivated Protein Kinase.Korean J PhysiolPharmacol.2010; 14(5): 299-304.
- Diep QN, Amiri F,Touyz RM, Cohn JS, Endemann D, Neves MF, Schiffrin EL. PPARa activator effects on Ang II-induced vascular oxidative stress andinflammation. Hypertension.2002; 40: 866-871.
- Chiou YL1, Shieh JJ, Lin CY. Blocking of Akt/NFkappaBsignaling by pentoxifylline inhibits plateletderivedgrowth factor-stimulated proliferation in Brown Norway rat airway smooth muscle cells. PediatrRes 2006; 60(6): 657-662.
- Gregory NE, Delaney A, Abdelmoaty S, Bas DB, Codeluppi S, Wigerblad G, Svensson CI.. Pentoxifylline and propentofylline prevent proliferation and activation of the mammalian target of rapamycinand mitogen activated protein kinase in cultured spinal astrocytes. J Neurosci Res. 2013; 91(2): 300-312.
- Peterson TC, Peterson MR, Robertson HA, During M, Dragunow M. Selective down-regulation of c-jun gene expression by pentoxifylline and c-jun antisense interrupts platelet-derived growth factor signaling: pentoxifylline inhibits phosphorylation of c-Jun on serine 73. MolPharmacol. 2002; 61(6): 1476-1488.
- Tsai TJ, Lin RH, Chang CC, Chen YM, Chen CF, KoFN, Teng CM.. Vasodilator agents modulate rat glomerularmesangial cell growth and collagen synthesis. Nephron 1995;70:91-99.
- Pinzani M, Marra F, Caligiuri A, DeFranco R, GentiliniA, Failli P, Gentilini P. Inhibition by pentoxifylline of extracellular signal-regulated kinase activation by platelet-derived growth factor in hepatic stellate cells. Br J Pharmacol 1996; 119(6): 1117-1124.
- Kavala AA, Aykut K, Sisli E, Kuserli Y, Ergur BU, Hazan E, Albayrak G. Pentoxifylline inhibits intimal hyperplasia and vascular smooth muscle cell proliferation in a rabbit carotid artery anastomosis model. African J Pharm Pharmacol2013; 7(14): 740- 748.
- Busk M, Mertz H, Espersen GT, Rasmussen K, MaengM. Effects of pentoxifylline on the vascular response to injury after angioplasty in rabbit iliac arteries. Basic Res Cardiol. 2008; 103(3): 257-264.