Research Article - Journal of Nutrition and Human Health (2017) Volume 1, Issue 1
Penicillin G's function, metabolites, allergy, and resistance.
- *Corresponding Author:
- Fadi Aldeek
Florida Department of Agriculture and Consumer Services Florida USA
Tel: (850) 617-7523
E-mail: fadi.aldeek@freshfromflorida.com
Accepted date: May 31, 2017
Citation: Canzani D, Aldeek F. Penicillin G?s function, metabolites, allergy, and resistance. J Nutr Hum Health. 2017;1(1):28-40.
DOI: 10.35841/nutrition-human-health.1.1.28-40
Visit for more related articles at Journal of Nutrition and Human HealthAbstract
The discovery of penicillin G was one of the most significant findings of the 20th century and certainly one of the most important discoveries in modern medicine. Since the 1940s, the world has remained dependent on this safe and effective antibiotic, and it has been used extensively not only in human medical applications, but in animal and agriculture to enhance the growth of livestock. Such diverse applications have resulted in the global distribution of this antibiotic, and its dispersal into a wide array of environments. Moreover, penicillin G is known to degrade into immunogenic metabolites, which have been identified in various agricultural products and wastewater systems, fuelling much debate on the safety of the use of this antibiotic. Unfortunately, the overuse of penicillin G and other antibiotics has led to the rise of resistant organisms and ushered us into a new age of treating infectious disease. The rise in resistance associated with penicillin G and its ability to produce allergy inducing metabolites in animal and medicinal applications are deeply intertwined, creating a unique and complex set of public health issues which is the subject of this review. Penicillin G will likely be used for many years to come, thus new understanding and insight is needed to address these public health issues.
Keywords
Penicillin G, Antibiotic resistance, Allergies, Metabolites, Public health, Antigenic determinants.
Introduction
Prior to antibiotics, bacterial infections such as pneumonia, gonorrhoea, and syphilis killed millions of people each year. Periodical outbreaks of bacterial disease filled hospitals and clinics, and with virtually no effective medical treatments available, patients suffered immensely [1]. Eventually, researchers discovered that certain chemicals could kill off or prevent the growth of bacteria, and today we possess more than 20 classes of antibiotics composed of dozens of commercially available drugs [2]. The first true antibiotic, penicillin, was discovered in 1928 by Alexander Fleming [3], and although over a decade passed before it became widely available, the discovery of penicillin marked a turning point in the way we treat disease.
Fleming, a professor of Bacteriology at St. Mary’s Hospital in London, had been studying the virulence of Staphylococcus in relation to colony morphology. After inoculating culture plates with Staphylococcus colonies, Fleming left for vacation and returned to find one plate contaminated with mold. Interestingly, bacterial colonies adjacent to the mold showed inhibited growth, and upon closer inspection, Fleming noticed these colonies were undergoing lysis [1,4]. It appeared that this mold, a strain of Penicillim notatum, contained an important anti-bacterial property, which Fleming named Penicillin [3]. It is reported that Fleming was frustrated by the difficulties in purifying penicillin and its lack of stability, but he saw the potential of the compound as a therapeutic, thus he continued to investigate and preserve his Penicillium molds, allowing other groups to build upon his work [1,5].
During World War II, the need for an antimicrobial agent became clear; armies were being devastated by casualties as the result of bacterial infections contracted during or after battle. Starting in 1939, Howard Florey and Ernst Chain at Oxford University began to investigate Penicillim notatum and are largely credited with transforming penicillin into the wonder drug it is regarded as today. By 1944, penicillin was being produced on an industrial scale in the US, allowing clinical trials to begin, and by the end of the 1940s, it was used to treat S. aureus infections in humans [1,6]. The success of penicillin spread by word-of-mouth, and public demand for the new drug began to rise. However, supplies of penicillin were still limited to treating wounded soldiers overseas [1]. Penicillin was regarded as a great success in treating allied soldiers, and in 1945, Fleming, Florey, and Chain were awarded the Nobel Prize in Medicine for their monumental achievements [4]. In 1946, penicillin was finally distributed to the open market, becoming the most profound contribution to improving human health in recorded history [4].
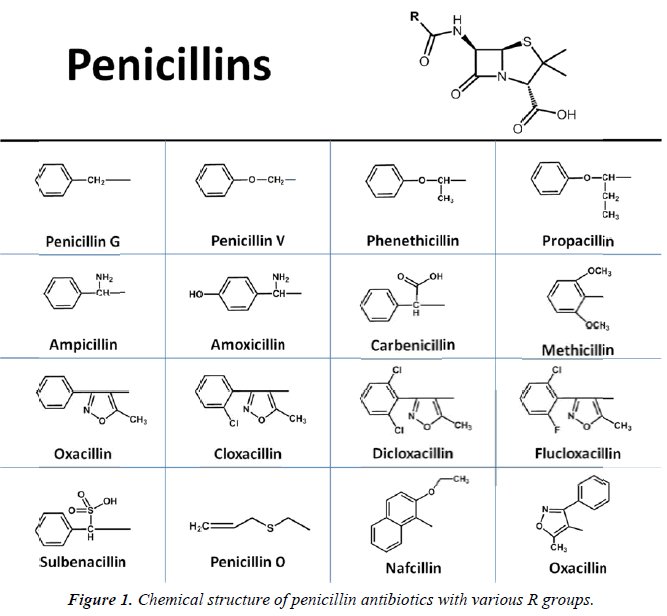
Florey et al. later determined that penicillin derived from two distinct strains Penicillium mold contained two unique penicillin molecule: one contained a benzyl ring (benzylpenicillin or penicillin G) and the other was substituted with a pentenyl group (2-pentenylpenicillin or penicillin F) [4]. It wasn’t until 1949 that Dorothy Crowfoot Hodgkin used X-ray crystallography to determine that each penicillin contained a β-lactam ring [7]. In 1959, both Batchelor and Sheehan used the molecular core or nucleus of penicillin, 6-aminopenicillanic acid, in the synthesis of penicillin [8,9]. Following this discovery, chemists began to create new synthetic penicillins simply by adding new side chains to 6-aminopenicillanic acid, generating new and effective antibiotics, including methicillin and pheneticillin by 1960 [10] (Figure 1). New subclasses of penicillins were synthesized during the “golden age” of antibiotic discovery between 1950 and the 1970s, including aminopenicillins, carboxypenicillins, and ureidopenicillins, and piperazine penicillins [11,12]. Other β-lactam antibiotics were also identified, including cephalosporins, monobactams, and carbapenems [13]. In addition, several completely new classes were discovered during this period, including the sulfonamides, aminoglycosides, tetracyclines, chloramphenicols, macrolides, glycopeptides, ansamycins, quiolones, steptogramins, and oxazolidones [14]. With all these new drugs entering the markets, many thought infectious disease would be eradicated. As a result, basic research into new antibiotics began to dwindle. In the decades that followed, no new classes of antibiotics were discovered, and all attempts at creating new drugs were done my modifying the existing antibiotics [15]. In 2015, teixobactin became the first antibiotic to be isolated from bacteria in decades, and appears to represent an entirely new class of compounds [16].
With each new antibiotic discovery, resistant organisms arose, limiting the effectiveness of the drugs, and calling into question the future of antibiotics. Organisms resistant to penicillin G and other β-lactam antibiotics were discovered in hospitals and clinics soon after their discoveries. After the discovery of β-lactamases, enzymes that are responsible for penicillin resistance by degrading the β-lactam ring, scientists realized it would be possible to inhibit these enzymes to counteract resistant bacteria. As early as the 1970s, inhibitors for β-lactamases were developed [17]. Inhibitors, such as tazobactam, clavulanic acid, and sulbactam, are available after being developed in the 1980s, and were shown to be effective in combination with certain β-lactam antibiotics. Recently, a non β-lactam β-lactamase inhibitor called avibactam has been shown to be highly effective in combination with cephalosporin antibiotics, and is now commercially available in the US and Europe [18,19]. However, the incredible adaptability of bacteria has also led to inhibitor-resistant bacteria [20]. Despite the growth of resistant organisms, penicillin G has remained one of the most widely used and least expensive antibiotics worldwide, and is one of the World Health Organization’s List of Essential Medicines [21]. If penicillin G retains its efficacy, it will be used for many years to come.
Penicillin and β-Lactam Function
The development of penicillins and their widespread applications in medicine necessitated a deeper understanding of the mechanisms behind their function. Early evidence suggested that penicillin G interfered with the bacterial cell wall in some way. From the beginning, it was clear that penicillin G worked on most Gram-positive bacteria, but only a few Gramnegative species. The major distinction between these two types of bacteria is the presence of an outer membrane in Gramnegative species, and thicker peptidoglycan (PG) cell wall in Gram-positive species. Examples of each include streptococci, staphylococci, and pneumococci for Gram-positive or gonococci and meningococci for Gram-negative species. Peptidoglycan is also known as murein, and is a polymer made of the monomer consisting of joined N-acetylglucosamine and N-acetylmuramic acid, with an amino acid pentapeptide bound to the N-acetylmuramic acid. The cell wall is formed when PG monomers are polymerized into long strands via glycosidic bonds. The strands are then cross-linked through the pentapeptide amino acid chains [22,23]. The synthesis of PG is catalyzed by penicillin binding proteins (PBPs) which feature glycosyl transferase and transpeptidase activity for polymerizing and crosslinking PG strands. The cross-linked PG is incredibly strong, and responsible for the structural integrity and shape of the cell, and is the prokaryotic cell’s most important defense feature [24].
Linear PG is synthesized one unit at a time, and commonly ends at the carboxy terminal of the pentapeptide chain with acyl-D-alanyl-D-alanyl. Penicillin is a structural analog of acyl- D-alanyl-D-alanyl, and due to this structural similarity, it may bind preferentially in the active site of the PG-transpeptidase that catalyzes the final step of the crosslinking reaction [25-27].
Specifically, the distances between the nitrogen atoms, N’ and N’, are identical (3.3 Å), and the distance between N’ and C’ are 5.4 Å and 5.7 Å for penicillin G and acyl-D-alanyl-D-alanyl, respectively. Furthermore, one of the conformations of acyl-Dalanyl- D-alanyl is almost identical to that of penicillin G, whose conformation is fixed by its ring system [25]. After penicillin binds to the active site of a PBP, the unstable β-lactam function breaks open as the result of nucleophilic attack, giving rise to an inactive penicilloyl-enzyme. More specifically, serine residues such as Serine 36 are conserved in several PBPs and have been shown to be the active site nucleophile. After nucleophilic attack, serine residues are acylated, which inhibits the PBP’s enzymatic function [28,29]. Without active PBPs, the cells aren’t able to form the cell wall, which best explains why only dividing cells are affected by penicillin G and other β-lactam antibiotics.
Penicillin G and its Metabolites
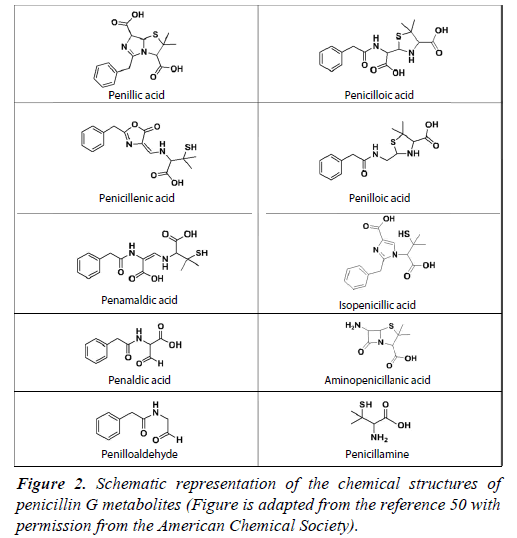
Upon binding to any PBP, the opening of the β-lactam function causes penicillin G to degrade into several biologically inactive metabolites, which may undergo subsequent rearrangements. These metabolites have been critically important in penicillin G use, as they are implicated in the potentially penicillin allergy. The four-membered β-lactam ring is present in all penicillins, and was identified in several other classes of antibiotics since the discovery of penicillin, including cephalosporins, monobactams, and carbapenems [30]. The β-lactam function is the key to the lethality of these antibiotics. Despite the efficacy of penicillin G and β-lactam antibiotics, there have been concerns over their stability. The four-membered β-lactam function exists under a tremendous amount of ring strain, which is the fundamental cause of its molecular instability and susceptibility to undergo ring opening. Importantly, this lack of stability contributes greatly to β-lactam’s reactivity and therefore, promotes antibiotic activity [31]. In penicillins, the highly labile β-lactam ring is fused to a five-membered thiazolidine ring at the 7 positions. The addition of the thiazolidine ring may be essential for functionality of the β-lactam ring because the fusion of these two features causes torsional rotation of the molecule, resulting in its non-planar structure, and contributes to large bond angles within the β-lactam ring. Thus, the already strained β-lactam ring is even more prone to cleavage when bound to thiazolidine [32]. However, in the case of monobactams, the sulfonate group bound to the nitrogen is electron withdrawing and activates the antibiotic by increasing the reactivity of the β-lactam ring [33]. The β-lactam ring is highly susceptible to hydrolytic opening under a wide range of conditions including auto-catalysis under neutral pH, and in the presence of acids, bases, heat, UV light, and enzymatic activity [34,35]. Upon ring opening, the penicillin G molecule undergoes rearrangements and forms several by-products known as metabolites (Figure 2). The most abundantly reported metabolites are penillic acid, penilloic acid, and penicilloic acid. In the presence of acids, penillic acid is the major product, with penicillenic acid, penicillamine, and penilloaldehyde also being produced from the hydrolysis of penicillin G’s amide function [36]. In alkaline conditions, penicilloic acid is known to be a major product, but it undergoes decarboxylation into penilloic acid [37-39]. While the molecule is most stable between pH 5 and 8, spontaneous fission and rearrangement of the β-lactam ring into an oxazolone ring and simultaneous cleavage of the thiazolidine ring may occur, resulting in penicillenic acid [40]. Due to stability issues in aqueous media, penicillin G should be kept in buffered solutions such as phosphate, acetate, or citrate, of which citrate buffer at pH 7 is the most effective at preventing degradation [41]. Penicillin G is most stable in a temperature range between 0°C to 52°C, above which it will rapidly degrade [42]. The degradation of penicillin G has been studied extensively in the past using a wide array of techniques, including the hydroxylamine method, UV-visible spectroscopy (UV-vis). Liquid chromatography has been a key technique used for this purpose, and it has been coupled to photodiode array detectors, UV-Vis detectors, and various types of mass spectrometers [41,43-46].
In any biological conditions, 6-aminopenicillanic acid may be formed by the removal of the acyl side chain contained within penicillin [47]. Furthermore, β-lactamases may cleave the β-lactam ring to produce α-methyl or α-ethyl benzyl penicilloate, or penicilloic acid. After penicillin G binds to penicillin binding proteins, it undergoes rearrangements, and forms a variety of metabolites. D-alanine carboxypeptidases can degrade penicillin G into phenyl acetyl glycine [48-50]. PBPs 5 and 6 of Escherichia coli can produce penicilloic acid and phenyl acetyl glycine, and the PBP may produce N-formyl penicillamine through a 3,3-dimethylthiazolinecarboxylate or related intermediate [51]. The metabolites of penicillin G do not possess antibacterial properties; however, they are known to form immunogenic conjugates with proteins in humans. Due to the widespread use of penicillin G in medicine, veterinary practices, and agricultural applications, metabolites formed from penicillin G may pose a significant risk to public health. As a result, identifying these metabolites has been of great interest to the scientific community. Penicilloic acid, 6-aminopenicillianic acid, and pnehoxyethyl penicilloic acid have been detected in excreted urine [47,52]. Later, seven of these metabolites were detected in human blood serum using high performance liquid chromatography-linear ion trap mass spectrometry. Five of the metabolites had not been detected in urine previously, and have the mass-to-charge (m/z) of 425, 427, 369, 529, and 575 [53].
For decades, penicillin G has been given to livestock including swine, poultry, beef and dairy cows to enhance animal growth, as a prophylactic, or to treat bacterial infections. Penicillin G residues have been identified in beef and pork samples from treated animals using liquid chromatography equipped with a UV detector at 215 nm [54,55]. When penicillin G is given to dairy cows, it will eventually end up in the milk. Lui used UHPLC-MS/MS to detect penilloic acid and penicilloic acid, which was present in 20% of the bovine milk samples tested, at an average concentration of 287 ng/mL and 320 ng/mL, respectively [56]. Additionally, Zhang used an enzyme-linked immunosorbent assay (ELISA) assay to detect penicilloic acid in adulterated milk that had been treated with penicillin G and treated with β-lactamase [49]. HPLC-triple quadrupole and timeof- flight mass spectrometry has also been used to detect many new penicillin G metabolites in cow’s milk, including potential amino acid-metabolite conjugates, such as phenylalaninepenicillamine [57,58]. Junza used UHPLC-MS/MS to determine that up to 28% of milk samples contained penicillin residues, and 11% were considered non-compliant with European Union regulations [59]. In another study, Junza determined that 44% of non-compliant milk samples contained penicillin G [60]. Given the susceptibility of penicillin G to degrade under heat, it is not surprising that penicillin G metabolites have been detected in pasteurized or thermally treated milk.
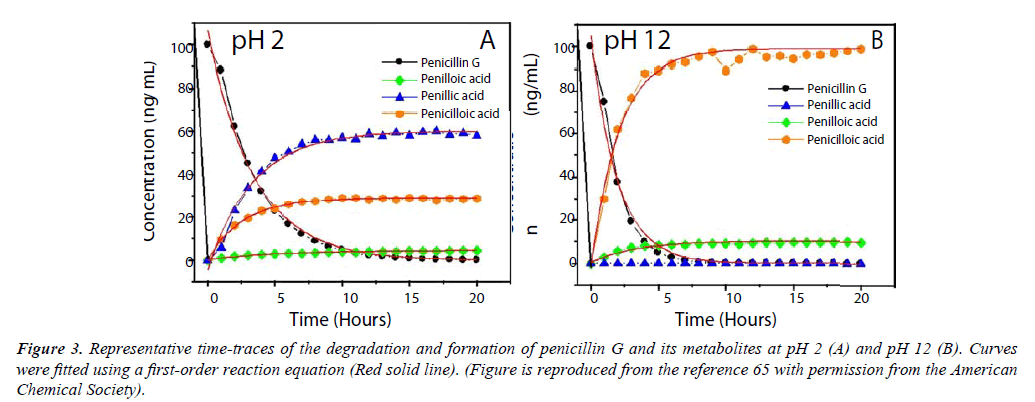
Citrus crops worldwide have been devastated by Huanglongbing (HLB), or citrus greening disease, which is caused by gramnegative bacteria in the genus Candidatus Liberibacter [61]. To overcome this economically devastating threat, several groups have initiated several research projects to combat infected citrus trees using penicillin G [62-64]. Although penicillin G was shown to be effective, there are some concerns over the stability of the molecule for this application. In order to better understand how penicillin would behave, our group has monitored the degradation of penicillin G in acidic and alkaline conditions at pH 2 and 12, respectively, using two distinct high-performance liquid chromatography-tandem mass spectrometry platforms (UHPLC-MS/MS). This analytical technique is recognized for its exceptional specificity and selectivity, and its advantages were well suited to characterizing the degradation of penicillin G. Under acidic conditions, penicillin G is shown to have a rate of degradation of 0.29 h-1 corresponding to a half-life of 2.4 hrs at pH 2 when the concentration of penicillin G was 100 ng/mL. Penicillin G was not detectable after approximately 10-15 hours [65]. Our UHPLC-MS/MS analysis of penicillin G’s degradation at pH 2 confirmed that penillic acid is the predominant species formed in acidic conditions, resulting in approximately 60% of the total metabolites (Figure 3A). Penicilloic acid is produced to some extent, resulting in almost 30% of the degradation products. The thiozolidine ring contained within penillic acid may rupture, forming isopenillic acid, which features a free thiol group [65]. Degradation in acidic media was of particular concern because if given orally, penicillin G would degrade tremendously in the stomach due to gastric acids. To resolve this problem, penicillin G is typically injected into the blood stream, or larger doses are given to maintain the drug’s efficacy [11]. Synthetic penicillins created after the discovery of penicillin G were designed to improve their molecular stability in acids, and may be taken orally in some cases [32]. At pH 12, our UHPLCMS/ MS data showed that penicilloic acid is the predominant metabolite formed, reaching almost 90% of the total metabolites produced in under 5 hours (Figure 3B). Penilloic acid was produced to some extent, and penillic acid was not detectable in alkaline conditions. When the concentration of penicillin G was 100 ng/mL, the rate of degradation was 0.51 h-1, corresponding to a half-life of only 1.35 hrs in basic conditions at pH.
Figure 3: Representative time-traces of the degradation and formation of penicillin G and its metabolites at pH 2 (A) and pH 12 (B). Curves were fitted using a first-order reaction equation (Red solid line). (Figure is reproduced from the reference 65 with permission from the American Chemical Society).
Penicilloic acid was rapidly formed to be the major product, but it undergoes decarboxylation into penilloic acid. Finally, we assessed the degradation of penicillin G in lemon matrix (approximately pH 4) over the course of one week. The metabolites produced were penillic acid, penilloic acid, and penicilloic acid. Penicillin G was not detectable after 4 days. Guided by these findings, we developed an extraction and analytical method to determine the concentration of penicillin G, penillic acid, and penilloic acid in citrus fruit and juice.
Using the Orbitrap and triple-quadrupole based UHPLCMS/ MS platforms, we analyzed a citrus fruit sample taken from a HLB infected tree that was treated with penicillin G; the presence of the metabolites penillic acid, isopenillic acid, penilloic acid, and penicilloic acid were confirmed. Penicillin G was not found in the citrus sample, however this was anticipated, as environmental conditions on the growing field, such as heat, UV radiation, and acidic conditions of the fruit would contribute heavily to degradation after treatment [65,66].
Recently, Dong et al. have shown this to be true by using liquid chromatography-single quadrupole mass spectrometry to identify penilloic acid, penicilloic acid, and isopenillic acid in a wastewater treatment plant and its receiving river. These metabolite species occupied 66%, 20%, and 13%, respectively, of the total concentration found in the river [67]. The presence of isopenillic acid was due to the conversion of penillic acid under alkaline or anaerobic conditions during the waste water treatment process. Compared to penillic acid, isopenillic acid features an open ring structure resulting in a free thiol group.
Penicillin Allergy
Antibiotics account for a small percentage of all adverse drug reactions. Although otherwise non-toxic, penicillin G is known to cause a wide range of allergic reactions, from skin rashes to anaphylactic shock and death. Treatment of penicillin G provokes anaphylaxis in approximately 1 in 5000 patients, which corresponds to relatively high morbidity; 75% of all anaphylactic deaths, approximately 500 to 1000 a year, are attributed to penicillin G [68,69].
By definition, the allergic reactions to penicillin G are immunogically mediated, and may be immediate or nonimmediate [70]. Of the four types of immunologically mediated drug reactions, i.e. Immunoglobin-E (IgE) mediated, antibody mediated, immune complex mediated, and T lymphocyte mediated, penicillin can cause all four [71]. Anaphylaxis is IgE mediated, and is the most serious of these reactions, occurring within 30 minutes to an hour of treatment [70]. The antigenic determinants which are the causative factors for these reactions have been well described, and are the haptenic metabolites which are produced from the degradation of penicillin G.
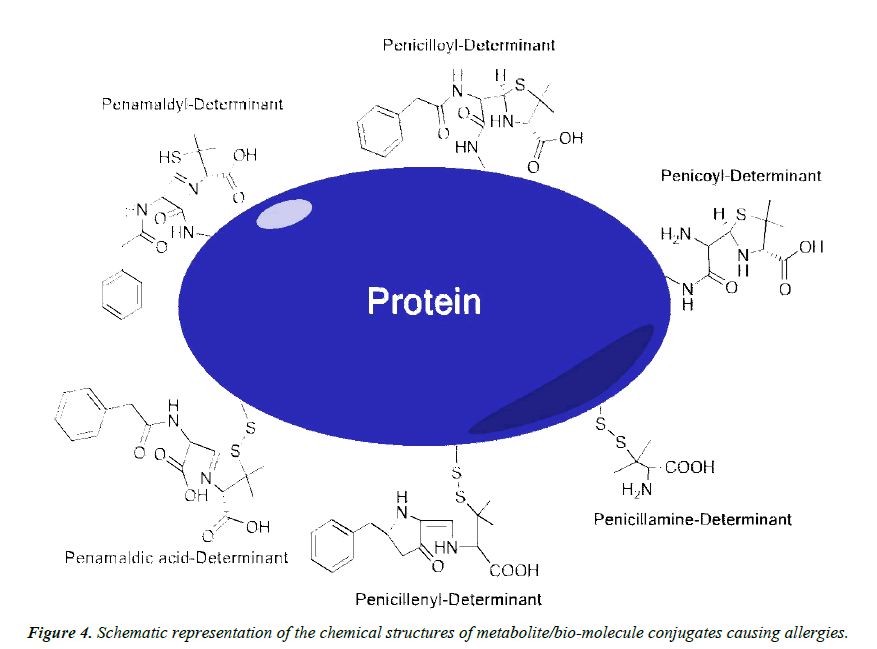
The causative agents of hypersensitivity to penicillin G are generally separated into two groups, and are known as the major and minor determinants (Figure 4). The major determinants provoke 90% to 95% of allergic reactions related to penicillin G, and penicilloyl-poly-L-lysine, and the penicilloyl component are the main metabolites of this group [71,72]. Major determinants are formed following the cleavage of penicillin G’s β-lactam ring, resulting in penicillenic acid. This metabolite is known to react quite readily with the ε-amino groups of extracellular lysine residues, forming the penicilloyl group via an amide bond [35,73]. Metabolites are thought to react and form amide bonds with the α-amino group at the N-terminus of insulin’s A chain, as well the ε-amino group of lysine residues on this molecule. It is also understood that ε-amino group of lysine-116 of the lysozyme molecule interacts with penicillin; however, it has been proposed that all lysine residues on lysozyme could be acylated [74]. Furthermore, penicilloyl groups were determined to bind to the blood serum albumin between methionine residues 123 and 297, and between methionine 297 and the C-terminal residue [75]. Thus, the majority of penicillin G allergies can be directly related to penicilloyl-lysine or penicilloyl-α-amino covalent interactions.
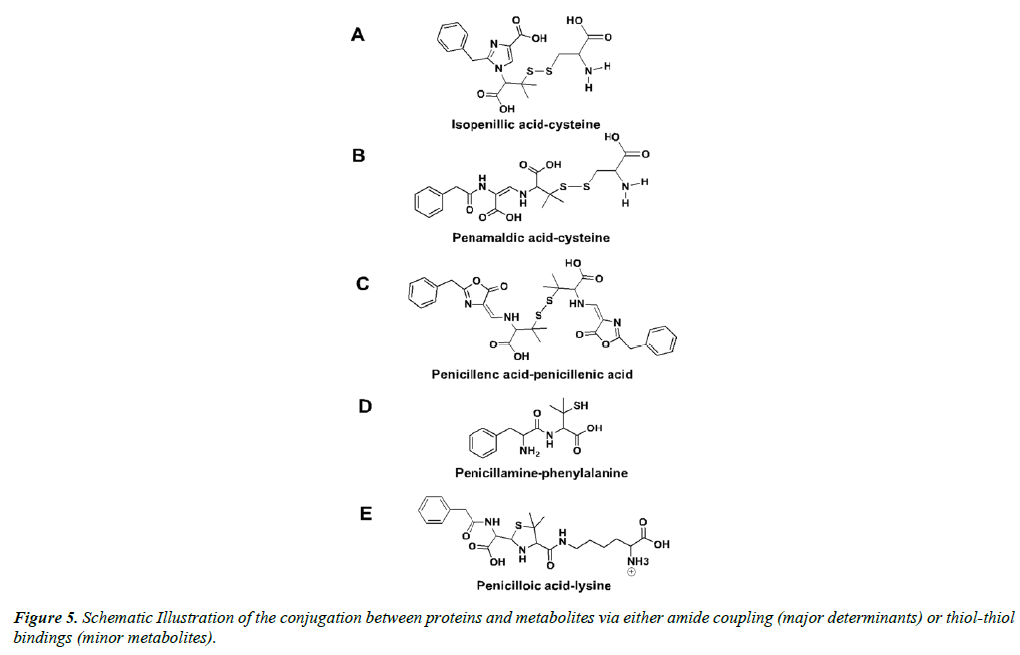
The other 5% to 10% of adverse allergic reactions to penicillin G are provoked by the so-called minor determinants, including the caboxylate functions of penicillamine, penicilloic acid, and penilloic acid, amongst others [71,76,77]. Largely, the minor determinants form hapten-carrier conjugates via disulfide bonds with cystine residues, including the penicillenyl-determinant and the penicillamine-determinant (Figure 4) [73]. It is worth noting that penicillin reacts rapidly with cystine residues, approximately 104 times faster than with alanine residues [78]. It is quite possible that the free thiol groups within isopenillic acid and other penamaldic derivatives could react with cystine residues as well (Figure 5A and 5B) [79]. Furthermore, disulfide metabolite-metabolite interactions occur, such as penicillenic acid disulfide, and could be the cause of an immune response (Figure 5C) [35]. Additional minor determinants may consist of polymerized penicillin G metabolites or penicilloic acid although these mechanisms have yet to be clarified [80]. Various other interactions between metabolites and free amino acids are likely; penicillamine-phenylalanine (Figure 5D), penicilloic acid-lysine (Figure 5E), and a serine-arginine-metabolite structure have been proposed [57].
The major and minor determinants of penicillin allergy have been used for some time in the form of a skin test to successfully predict penicillin hypersensitivity in patients. This first-line method uses a major determinant mixture containing benzylpenicilloyl-poly-lysine and a minor-determinant mixture, containing various penicillin metabolites, to test for IgE mediated immune responses [68,81,82]. These tests can only detect IgE interactions, but because IgE mediated responses are the most serious and can cause anaphylaxis and death, skin testing has the potential to save countless lives each year. Using the major determinant mixture, approximately 99% of patients who test negative for IgE reactions will safely tolerate penicillin G with no consequential reaction [83]. For patients who test positive for an IgE response, desensitization to penicillin G has been established as an effective medical procedure, for which several protocols are now available [84,85]. Desensitization involves giving increasing doses of penicillin to a susceptible patient, starting with a dose approximately 1/10,000 of the typical recommended dose for treatment [68]. Although desensitization has been around for some time, it is not a well understood phenomena [8]. During desensitization, patients must be monitored, as certain complications can arise, such as anaphylaxis [86].
Bacterial Resistance
In the same year Alexander Fleming won the Nobel Prize for his discovery of penicillin, he cautioned that resistant bacteria would arise if the drug was misused [91]. True to his word, penicillin resistant bacteria were discovered throughout the late 1940s and into the 1950s, with S. aureus being among the first [92]. Since then, bacteria have continued to develop resistance at a rate that outpaces the discovery of new antibiotics. Although antibiotics may be our most important tools for treating infectious disease, some resistance has been reported for all of these drugs. It is apparent that we have moved into a new age where resistance to these drugs is a major threat to public health that results in millions of human hospitalization and over 20,000 deaths every year [88,93].
There are generally three mechanisms by which an organism acquires resistance to an antibiotic agent; random genetic mutation and recombination, horizontal gene transfer, and through artificial selective pressure through the use of the antibiotic drugs. Antibiotic resistance can also be intrinsic, or through functional or structural characteristics of a bacteria [94]. Genes that confer bacterial resistance are referred to as antibiotic resistance genes (ARGs) and are present in bacterial genomes or as free plasmids. Such ARGs may persist and spread throughout the environment through bacterial cell replication and multiplication. One crucial mechanism for the evolution of ARGs arise though random genetic mutation and recombination [95]. Horizontal gene transfer, also known as lateral gene transfer, is a process by which bacteria share genetic information, in some cases ARGs [95]. Horizontal gene transfer has been known for over half a century, and is one of the main drivers of bacterial adaptation and resistance to antimicrobial agents [96,97]. Instances of ARG transfer have been heavily implicated in the transmission of resistant bacteria in humans [97]. Such practices impart immense selective pressure that drives and accelerates the rate at which resistant evolves. Random mutation events that lead to resistance are rare and slow, and multiple mutations may be necessary in combination to produce an effective resistance mechanism. However, when humans are applying such immense selective pressure through the use of antibiotics, random genetic mutations will accumulate much faster than in environmental conditions, and thus resistant organisms are arise quickly [95]. When an ARG arises, it will give the organism the ability to survive and replicate after treatment of an antibiotic. Surviving bacteria are free to reproduce and therefore, the buildup of resistant organisms over time is inevitable. The combination of random mutations, gene transfer, and selective pressure imposed by human use of antibiotics have worked simultaneously to accelerate the rise of resistant organisms, leading to the public health crisis we have found ourselves in today.
The use of penicillin G and other β-lactam antibiotics has selected for β-lactamases, enzymes that cleave the β-lactam ring, rendering the antibiotic inactive. The first β-lactamase (penicillinase) was discovered in Staphylococci aureus in 1944, and since then, over 400 types of β-lactamases have been discovered in both Gram positive and Gram-negative species [98,99]. These enzymes likely evolved from random mutations to penicillin binding proteins, which facilitate cell wall synthesis and degradation [99,100]. Resistant S. aureus, equipped with β-lactamases were first identified in British hospitals in 1948 [101]. Over time, many species acquired resistance through reduced affinity PBPs including Streptococcus pneumoniae and Neisseria meningitides [102,103].
Although it was once the first-choice and most effective treatment for gonorrhoea, the global rise of chromosomally mediated resistant Neisseria gonorrhoeae has severely limited penicillin G’s role in treating this sexually transmitted infection [104,105]. An alternative mechanism for penicillin G resistance in some Gram-negative species is decreased number of porins. Porins are protein channels that are embedded in the outer membrane of Gram-negative bacteria that act as channels for nutrients and waste products to enter or exit the bacteria [106]. Porins also allow certain antibiotics to enter into cells, including penicillin G. In some strains of E. coli, reduced numbers of porins have been discovered, reducing the sensitivity of the bacteria to penicillin G [107].
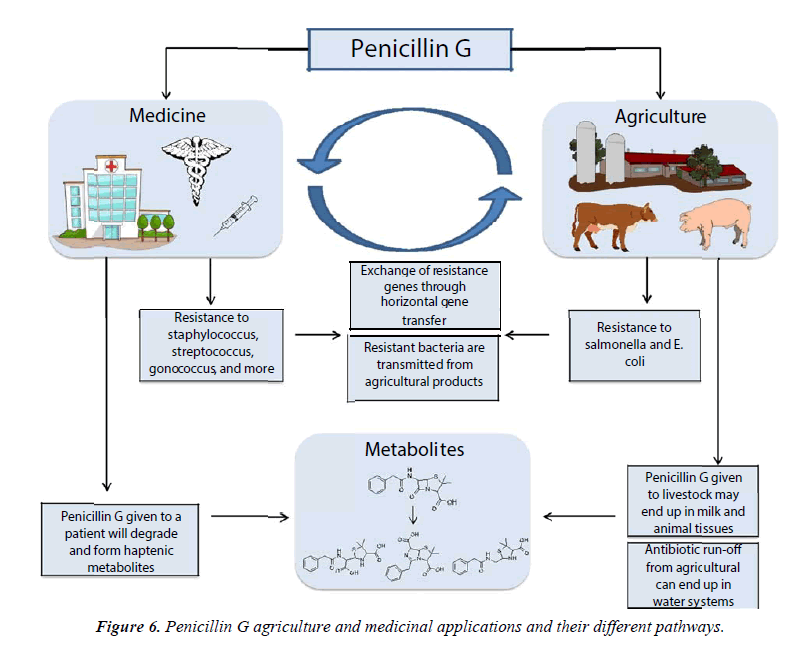
It is estimated that up to 80% of antibiotics sold in the United States are used in agriculture [108,109]. Primarily, they are given to livestock to treat infection, prevent infection, or to accelerate and enhance growth [110]. 50% of the antibiotics used in animal are given as growth promoters, and of this, penicillin G accounts for 55% to 60% of use [111,112]. This practice, which has been used since the 1950s, continuously introduces low-dose antibiotics into otherwise healthy populations of animals. Such immense selective pressure has been pivotal in the development of resistant organisms. Indeed, antibiotic resistant bacteria have been found in livestock heavily treated with antibiotics [113].
While there has been some debate over the extent of this problem, considerable evidence suggests that antibiotic resistance related to animal use can spread to inflict illness on the human population [114]. For instance, antibiotic resistant bacteria have been found in food products related to antibiotictreated animals; thousands of hospitalizations have occurred from 1973 to 2011 as the result of Salmonella outbreaks in beef products, many of which contained multi-drug resistant Salmonella strains [115]. Additionally, an outbreak of multidrug resistant Salmonella Heidelberg, which affected 136 patients in 34 states, was traced back to consumption of ground turkey [116]. Overall, the US Center for Disease Control has determined that 1 in 5 of all resistant infections are transmitted from food and animals [117].
Approximately 36 metric tons of a variety of antibiotics are used in horticulture each year [118] to treat bacterial infections in crops. For infections such as fire blight in apples or pears, antibiotics remain the most practical and cost-effective method for treatment [119]. Directly after application of antibiotics to crops, the concentration of free antibiotics in soil has been found to be as high as 12 μg/mL, which is theoretically high enough to select for clinically relevant levels of resistance. However, photodegradation, adsorption to soil, dilution by rain, and irrigation reduce the antibiotic concentrations, and antibiotics may not be found in soil several days after application [118]. Others have pointed out that plants and humans do not typically share pathogens [120], although it is a possibility for resistance to be transferred to clinically relevant species via ARGs and horizontal gene transfer. As such, the effects on resistance from antibiotic use in horticulture are considered by many to be negligible in comparison to the effects antibiotic use on animals. Compared to other antibiotics, penicillin G has not been widely used in horticulture. As mentioned earlier in this review, penicillin G has been used in various research projects to combat the citrus greening disease. While research shows the promising use of penicillin G to suppress the HLB bacterial disease in citrus infected trees, there are still many factors to be taken into consideration before this antibiotic is regulated by government agencies. However, it is important to note that the risk for penicillin G to contribute to resistance should be considered inherently lower than other antibiotics when used in horticulture due to their high susceptibility to degradation (Figure 6).
Actions have been taken to limits the use of penicillin and other antibiotics in animal in the European Union and in the United States. Between 2010 and 2011, every EU country, with the exception of Spain, reduced its agricultural use of antibiotics. This trend was led by Denmark, where 11 different antibiotics were voluntarily given up by farmers through the 1990s. Despite stopping or limiting antibiotic use, Denmark reported an increase of 60% in the number of pigs produced during this time [121]. In 2013, the US Food and Drug Administration issued a voluntary guidance to industry to limit the use of medically important antibiotics in animal. Although this guidance is symbolically important, many predict the guidance will not be enough to deter antibiotic use in agriculture [122].
Conclusion
The widespread use of penicillin G since the 1950s has led to several important public health concerns (Figure 6). Heavy use in medicine and animal to treat infectious disease for many years led to the rise of resistant bacteria, which has had serious repercussions. Outbreaks of resistant organisms kill thousands each year. Furthermore, penicillin G allergies, which are caused by its degradation products, cause the majority of anaphylactic allergic responses among all antibiotics. Penicillin G skin testing and desensitization protocols have cut down on the number of deaths related to penicillin G use. However, the prevalence of the use this antibiotic in animal leads to the possible contamination of food products, which has been the subject of debate for decades. Recent advances in analytical techniques, including UHPLC-MS/MS, have provided significant improvements in our abilities to identify penicillin G and its metabolites in various food products and environmental applications. We believe that this review will help us understand the problems associated with penicillin use, including the threat of possible allergic reactions, and the growth bacterial resistance for future generations to come.
Acknowledgments
This review is a dedication to the soul of the mother of Dr. Fadi Aldeek. Noufa Abdelkader Hamad passed away on July 28th 2012. In her last days, Noufa Abdelkader Hamad suffered from a rare bacterial infectious disease called Nectrotizing Fasciits. Penicillin G and other antibiotics at different doses were given to her to stop or slow the progression of the infection. After a difficult period of 6 months fighting the disease, she developed an antibacterial resistance to these antibiotics, which led to a rapid progression of the infection and caused her death. May her soul rest in peace, “I love you Mom.” We believe that the information provided in this manuscript will benefit the scientific community and allow a better understanding of the mechanism, metabolites, and impact on public health of penicillin G once administrated in humans.
References
- The Discovery and Development of Penicillin. In Society, A. C., Ed. ACS Publications: 1999.
- Coates AR, Halls G, Hu Y. Novel classes of antibiotics or morae of the same?Br J Pharmacol.2011;163:184-94.
- Fleming A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenzæ. Br J Exp pathol. 1929;10:226-36.
- Kong KF, Schneper L, Mathee K. Beta-lactam antibiotics: From antibiosis to resistance and bacteriology. APMIS.2010;118:1-36.
- Hare R. New light on the history of penicillin. Med Hist.1982;26:1-24.
- Abraham EP, Chain E, Fletcher CM, et al.Further observations on Penicillin. The Lancet. 1940;238:177-89.
- Hodgkin DC.The X-ray analysis of the structure of penicillin. Adv Sci.1949;6:85-9.
- Sheehan JC, Logan KRH. A general synthesis of the penicillins. JAmChem Soc. 1959;81:5838-9.
- Batchelor FR, Doyle FP, Nayler JH, et al. Synthesis of Penicillin: 6-aminopenicillanic acid in penicillin fermentations. Nature.1959;183:257-8.
- Fairbrother RW, Taylor G.Sodium methicillin in routine therapy. Lancet. 1961;1:473-6.
- Antimicrobe, drugs. Penicillins. Available from: http://www.antimicrobe.org/d24.asp
- Aminov RI. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front Microbiol.2010;1:134.
- Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clinmicrobiolrev. 2010;23:160-201.
- Walsh W. Introduction: Antibiotic Resistance. Chem Rev.2005;105:391-4.
- Coates A, Hu Y, Bax R, et al. The future challenges facing the development of new antimicrobial drugs. Nat Rev Drug Discov.2002;1:895-910.
- Ling LL, Schneider T, Peoples AJ, et al. A new antibiotic kills pathogens without detectable resistance. Nature.2015;517:455-9.
- Brown AG, Butterworth D, Cole M, et al. Naturally-occurring beta-lactamase inhibitors with antibacterial activity. J Antibiot. 1976;29:668-9.
- Wang DY,Abboud MI, Markoulides MS, et al. The road to avibactam: The first clinically useful non-beta-lactam working somewhat like a beta-lactam. Future Med Chem.2016;8:1063-84.
- Ehmann DE, Jahic H, Ross PL, et al. Avibactam is a covalent, reversible, non-beta-lactam beta-lactamase inhibitor. Proc Natl Acad Sci. 2012;109:11663-8.
- Drawz SM, Papp-Wallace KM, Bonomo RA. New β-lactamase inhibitors: Atherapeutic renaissance in an MDR world. Antimicrob Agents Chemother. 2014;58:1835-46.
- World Health Organization. WHO model list of essential medicines. In Organization, W H Ed. 2013;18.
- Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMSMicrobiol Rev.2008;32:149-67.
- Hayhurst EJ, Kailas L, Hobbs JK, et al. Cell wall peptidoglycan architecture in Bacillus subtilis. Proc Natl Acad Sci.2008;105:14603-8.
- Typas A, Banzhaf M, Gross CA, et al. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol.2012;10:123-36.
- Tipper DJ, Strominger JL. Mechanism of action of penicillins: A proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci USA.1965;54:1133-41.
- Wise EM, Park JT.Penicillin: Its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci USA.1965;54:75-81.
- Babington R, Matas S, Marco MP, et al. Current bioanalytical methods for detection of penicillins. AnalBioanal Chem.2012;403:1549-66.
- Yocum, RR, Waxman DJ, Rasmussen JR, et al. Mechanism of penicillin action: Penicillin and substrate bind covalently to the same active site serine in two bacterial D-alanine carboxypeptidases. Proc Natl Acad Sci USA.1979;76:2730-4.
- Yocum RR, Rasmussen JR,Strominger JL. The mechanism of action of penicillin. Penicillin acylates the active site of Bacillus stearothermophilus D-alanine carboxypeptidase. J Biol Chem.1980;255:3977-86.
- Poole K. Resistance to beta-lactam antibiotics. Cell Mol Life Sci.2004;61:2200-23.
- Cohen NC.Beta-Lactam antibiotics: Geometrical requirements for antibacterial activities. J MedChem.1983;26:259-64.
- Deshpande A, Baheti K, Chatterjee N. Degradation of b-lactam antibiotics. Curr Sci.2004;87:1684-95.
- https://books.google.com/books?id=v8DSBQAAQBAJ&pg=PA374&lpg=PA374&dq=electron+ withdrawing+groups+on+monobactams&source=bl&ots=jZ35qFSK5&sig=QpAo9HuK0nA0UgQJRLYW4drYZkU&hl=en&sa=X&ved=0ahUKEwivmuKmwNvSAhVM6GMKHXgSBwkQ6AEIRDAH#v=onepage&q=electron%20withdrawing%20groups%20on%20monobactams&f=false
- Robinson FA. The chemistry of Penicillin. Clarke HT, Johnson JR, Robinson R, editors. Pp. 1042 and Appendix. Princeton University Press, New Jersey (London: Geoffrey Cumberlege) 1949, £9 9s. 0d. JPharm Pharmacol.1949;1:634-5.
- Schwartz MA. Chemical aspects of penicillin allergy. J Pharm Sci.1969;58:643-61.
- Schwartz MA. Mechanism of degradation of penicillin G in acidic solution. JPharm Sci.1965;54:472-3.
- Schneider CH, De Weck AL. A new chemical aspect of penicillin allergy: The direct reaction of penicillin with epsilon-amino-groups. Nature.1965;208:57-9.
- Hou JP, Poole JW. β-lactam antibiotics: Their physicochemical properties and biological activities in relation to structure. JPharm Sci.1971;60:503-32.
- Blaha JM, Knevel AM,Kessler DP, et al. Kinetic analysis of penicillin degradation in acidic media. JPharm Sci.1976;65:1165-70.
- SammesPG. Recent chemistry of the β-lactam antibiotics. Chem Rev.1976;76:113-55.
- Lu X, Xing H, Su B, et al. Effect of buffer solution and temperature on the stability of penicillin G. JChemEng Data.2008;53:543-47.
- Kheirolomoom A,Kazemi-Vaysari A,Ardjmand M, et al. The combined effects of pH and temperature on penicillin G decomposition and its stability modeling. ProcBiochem.1999;35:205-11.
- Batchelor FR,Chain EB,Hardy TL, et al.6-Aminopenicillanic acid.III. Isolation and purification. Proc R Soc Lond B Biol Sci USA.1961;154:498-508.
- NavarroPG,BlázquezIH, Osso BQ, et al. Penicillin degradation catalysed by Zn(II) ions in methanol. IntJBiol Macromol.2003;33:159-66.
- Ania C, Pelayo J,Bandosz T. Reactive adsorption of penicillin on activated carbons. Adsorption.2011;17:421-9.
- Ren Z, Zhang W, Li J, et al. Effect of organic solutions on the stability and extraction equilibrium of Penicillin G. JChemEng Data.2010;55:2687-94.
- Cole M, Kenig MD, Hewitt VA. Metabolism of penicillins to penicilloic acids and 6-aminopenicillanic acid in man and its significance in assessing penicillin absorption. AntimicrobAgent Chemother.1973;3:463-8.
- Knott-Hunziker V,Petursson S, Waley SG, et al. The acyl-enzyme mechanism of β-lactamase action. The evidence for class C β-lactamases. Biochem J.1982;207:315-22.
- Zhang Y, Jiang Y, Wang S. Development of an enzyme-linked immunosorbent assay to detect benzylpenicilloic acid, a degradation product of Penicillin G in adulterated milk. J AgricFood Chem.2010;58:8171-5.
- Hammarström S, Strominger JL. Degradation of penicillin G to phenylacetylglycine by D-alanine carboxypeptidase from Bacillus stearothermophilus. Proc NatlAcadSciUSA.1975;72:3463-7.
- Amanuma H, Strominger JL. Simultaneous release of penicilloic acid and phenylacetyl glycine by penicillin-binding proteins 5 and 6 of Escherichia coli.J Bacteriol.1984;160:822-3.
- Birner J. Determination of phenoxymethyl penicilloic acid and phenoxyethyl penicilloic acid in urine in the presence of the parent penicillins.J Pharm Sci.1970;59:757-60.
- Ho HP, Lee RJ, Chen CY, et al. Identification of new minor metabolites of penicillin G in human serum by multiple-stage tandem mass spectrometry. RapidCommun Mass Spectrom.2011;25:25-32.
- Moats WA, Romanowski RD. Multiresidue determination of beta-lactam antibiotics in milk and tissues with the aid of high-performance liquid chromatographic fractionation for clean-up. JChromatogra.1998;812:237-47.
- Moats WA, Romanowski RD. Determination of penicillin G in beef and pork tissues using an automated LC clean-up. J AgriFood Chem.1998;46:1410-3.
- Liu C, Wang H, Jiang Y, et al. Rapid and simultaneous determination of amoxicillin, penicillin G, and their major metabolites in bovine milk by ultra-high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B AnalTechnol Biomed Life Sci.2011;879:533-40.
- Junza A, Barbosa S, Codony MR, et al. Identification of metabolites and thermal transformation products of quinolones in raw cow's milk by liquid chromatography coupled to high-resolution mass spectrometry. J Agric Food Chem.2014;62:2008-21.
- Li L, Guo C, Ai L, et al. Research on degradation of penicillins in milk byβ-lactamase using ultra-performance liquid chromatography coupled with time-of-flight mass spectrometry. J Dairy Sci.2014;97:4052-61.
- Junza A,Dorival-García N,Zafra-Gómez A, et al. Multiclass method for the determination of quinolones and β-lactams, in raw cow milk using dispersive liquid-liquid microextraction and ultra-highperformance liquid chromatography-tandem mass spectrometry. J Chromatography A.2014;1356:10-22.
- Junza A,Amatya R,Barron D, et al. Comparative study of the LC-MS/MS and UPLC-MS/MS for the multi-residue analysis of quinolones, penicillins and cephalosporins in cow milk,27and validation according to the regulation 2002/657/EC. J Chromatogr B Anal Technol BiomedLife Sci.2011;879:2601-10.
- Nageswara-Rao M, Irey M, Garnsey SM, et al. Candidate gene markers for Candidatus Liberibacterasiaticusfor detecting citrus greening disease. J Biosci. 2013;38:229-37.
- Zhang M, Powell CA, Guo Y, et al. Characterization of the microbial community structure in Candidatus Liberibacterasiaticus-infected citrus plants treated with antibiotics in the field. BMC Microbiol.2013;13:112.
- Zhang M, Powell CA, Zhou L, et al. Chemical compounds effective against the citrus Huanglongbing bacterium 'Candidatus Liberibacterasiaticus' in planta. Phytopathology.2011;101:1097-103.
- Bovxe MJ. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. JPlant Pathol.2006;88:7-37.
- Aldeek F, Canzani D, Standland M, et al. Identification of penicillin G metabolites under various environmental conditions using UHPLC-MS/MS. J Agric Food Chem.2016.
- Aldeek F, Rosana MR, Hamilton ZK, et al. Method for the determination and quantitation of penicillin G and its metabolites in citrus fruits affected by huanglongbing. J Agric Food Chem.2015;63:5993-6000.
- Li D,Yang M, Hu J, et al. Determination of penicillin G and its degradation products in a penicillin production wastewater treatment plant and the receiving river. Water Res.2008;42:307-17.
- Arroliga ME, Pien L. Penicillin allergy: Consider trying penicillin again. Cleve Clin J Med.2003;70:313-4, 317-8, 320-1 passim.
- Gruchalla RS,Pirmohamed M. Antibiotic allergy. NEnglJMed.2006;354:601-9.
- BousquetPJ,Kvedariene V, Co-Minh HB,et al. Clinical presentation and time course in hypersensitivity reactions to β-lactams. Allergy.2007;62:872-6.
- Frumin J,Gallagher JC. Allergic cross-sensitivity between penicillin, carbapenem, and monobactam antibiotics: what are the chances? Ann Pharmacother.2009;43:304-15.
- Adkinson NF,Thompson WL,Maddrey WC, et al. Routine use of penicillin skin testing on an inpatient service. N EnglJMed.1971;285:22-24.
- Weltzien HU, Padovan E. Molecular features of penicillin allergy. J Invest Dermatol.1998;110:203-6.
- Corran PH, Waley SG. The reaction of penicillin with proteins. Biochem J.1975;149:357-64.
- Lafaye P,Lapresle C. Identification of two fixation sites for penicilloyl groups on the albumin molecule from penicillin-treated patients. FEBS Letters.1988;234:305-8.
- Levine BB. Studies on the immunological mechanisms of penicillin allergy: I. Antigenic specificities of guinea-pig skin sensitizing rabbit anti-benzylpenicillin antibodies. Immunology.1964;7:527-41.
- Rodriguez-Bada JL, Montanez MI, Torres MJ, et al. Skin testing for immediate hypersensitivity to betalactams: Comparison between two commercial kits. Allergy.2006;61:947-51.
- Nakken KF, Eldjarn L, Pihl A. The mechanism of inactivation of penicillin by cysteine and other mercaptoamines. Biochem Pharmacol.1960;3:89-100.
- Navarro PG,Osso BQ,Ortiz RG, et al. Inhibition of β-lactamase II of Bacillus cereus by penamaldic derivatives of Penicillins.Antimicrob Agent Chemother.2004;48:1058-60.
- Dewdney JM, Edwards RG. Penicillin hypersensitivityis milk a significant hazard? A review. J R Soc Med.1984;77:866-77.