Brief Report - Journal of Gastroenterology and Digestive Diseases (2023) Volume 8, Issue 1
Patient perspectives on switching from intravenous to subcutaneous infliximab and vedolizumab in inflammatory bowel disease: Results from a cross-sectional survey.
Lotte Oldenburg#, Anouk M Wijnands#, Bas Oldenburg*Department of Gastroenterology and Hepatology, Inflammatory Bowel Disease Center, University Medical Center Utrecht, Utrecht, the Netherlands
- *Corresponding Author:
- Oldenburg B
Department of Gastroenterology and Hepatology
Inflammatory Bowel Disease Center
University Medical Center Utrecht
Utrecht, the Netherlands
Tel: +31 88 75 57 325
E-mail: boldenbu@umcutrecht.nl
Received: 12-May-2022, Manuscript No. JGDD-22-60517; Editor assigned: 14-May-2022, PreQC No. JGDD-22-60517(PQ); Reviewed: 30-May-2022, QC No JGDD-22-60517; Revised: 06-Jul-2022, Manuscript No. JGDD-22-60517(R); Published: 13-Jul-2022, DOI:10.35841/jgdd-7.7.131
Citation: Oldenburg L, Wijnands AM, Oldenburg B. Patient perspectives on switching from intravenous to subcutaneous infliximab and vedolizumab in inflammatory bowel disease: Results from a cross-sectional survey. J Gastroenterology Dig Dis. 2022;7(7):131
Abstract
Objective: Recently, Subcutaneous (SC) formulations of Infliximab (IFX) and Vedolizumab (VDZ) were approved for clinical use. The present study aims to explore whether patients with Inflammatory Bowel Disease (IBD), receiving Intravenous (IV) IFX or VDZ therapy, prefer IV or SC routes of administration. Methods: We conducted a cross-sectional web-based survey on the willingness to switch from IV to SC administration among IBD patients, treated with IV IFX or VDZ in a large tertiary hospital in the Netherlands. All adult IBD patients (including Crohn’s disease, ulcerative colitis, or IBD-unclassified) visiting the outpatient department for IV administration of IFX or VDZ between June and October 2021 were asked to participate. Predictors for willingness to switch to SC injections were identified by logistic regression analysis. Result: In total, 148 out of 219 patients who agreed to receive the questionnaire, gave informed consent and completed the questionnaire (response rate 68%). Seventy-nine patients (53%) were willing to switch to SC formulations. The most frequently cited reasons for willingness to switch were dependency on hospital-based care and travel time-related issues. Lower treatment satisfaction rates (Likert scale 0-10) and receiving IV therapy <1 year were associated with willingness to switch in, respectively, univariable and both univariable and multivariable analysis. Conclusion: Fifty-three percent of patients with IBD receiving IV therapy is willing to switch to SC injections. Predictors for willingness to switch included lower treatment satisfaction rates (only univariable analysis) and <1 year of IV therapy.
Keywords
Patient preferences, Subcutaneous, Intravenous, Ulcerative colitis.
List of abbreviations
CD = Crohn’s Disease; CI = Confidence Interval; IBD = Inflammatory Bowel Diseases; IFX = Infliximab; IQI = Interquartile Interval; IV = Intravenous; OR = Odds Ratio; SC = Subcutaneous; TNF = Tumour Necrosis Factor; UC = Ulcerative Colitis; VDZ = Vedolizumab.
Introduction
Both the Tumour-Necrosis-Factor (TNF) Alpha Inhibitor Infliximab (IFX) and the integrin antagonist Vedolizumab (VDZ) are highly effective in the treatment of Ulcerative Colitis (UC) and Crohn’s Disease (CD). VDZ and IFX, are routinely administered Intravenously (IV), but recently, Subcutaneous (SC) options became available for both compounds [1-3]. As maintenance therapy the efficacy and safety profile of SC administration compared with IV formulations is equal for VDZ and non-inferior for IFX, also the SC and IV formulations have comparable safety profiles [4,5].
SC administration of biological might have considerable advantages as compared to IV dosing schemes. In addition to the theoretical advantage of a reduced immunogenicity [4], SC administration will lead to a transition from hospital-to homebased care and therefore to a reduction in clinical requirements and related healthcare costs [6]. In addition, it could reduce dependency on others, enhance the feeling of being in control, potentially translating in a higher quality of life. On the other hand, switching from an IV to SC formulation might lead to feelings of apprehension and anxiety, and potentially to a nocebo effect in patients [7].
The aim of the present study is to assess the willingness of to switch from IV to SC administration in IBD patients, and to identify reasons for preferring a switch of formulation.
Methods
Study population
All adult IBD patients (CD, UC, or IBD-unclassified) receiving IV IFX or VDZ at the out-patient clinic of the Utrecht University Medical Centre in the Netherlands between June 2021 and October 2021 were approached to participate in this cross-sectional questionnaire study.
Questionnaire
Questions included satisfaction with current IV therapy (Likert scale 0 to 10), duration of IV therapy, total travel time, and reasons for switching to SC therapy or continuation of IV therapy. Patient demographics, diagnosis and medication history were extracted from electronic health records. Clinical remission (categorized as yes or no) was based on the most recent status report from the treating physician or nurse documenting information on presence or absence of IBDrelated symptoms.
Statistical analysis
Descriptive statistics were used to describe baseline characteristics and questionnaire results. Continuous data were presented as mean with standard deviation or medians with interquartile interval (IQI), as appropriate. Categorical variables were summarized with frequencies.
Predictors for willingness to switch were examined by univariable and multivariable logistic regression analyses. Assumptions for logistic regression were checked. The assumption of linearity was violated for disease duration and therefore this variable was categorized. The following variables were included in the regression analyses, based on current literature and expert opinion: age (per year increase), duration of current IV therapy (<1 and ≥ 1 year), satisfaction with current IV therapy, total travel time (<60 and ≥ 60 minutes), disease in clinical remission, IBD disease duration (<10 and ≥ 10 years), and prior biological therapy usage [8]. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using R statistical software (stats and car packages), version 4.0.3.
Ethical considerations
The institutional review board assessed the study as not subject to the Medical Research Involving Human Subjects Act. Informed consent was given by all patients.
Results
Study population
In total, 148 out of 219 patients that agreed to receive the questionnaire, gave informed consent and completed the questionnaire (response rate 68%, 79 women (53%), median age 40 years (IQI 26-51)). Most patients had a diagnosis of CD (74%). Sixty-five percent of patients were on IFX therapy while 35% received IV VDZ. Baseline characteristics are presented in Table 1.
| Characteristics | Patients (N=148) |
|---|---|
| Female sex, n(%) | 79 (53) |
| Age (y), median (IQR) | 40 (26-51) |
| IBD type, n (%) | |
| Crohn’s disease | 110 (74) |
| Ulcerative colitis | 35 (24) |
| IBD unclassified | 3 (2) |
| Age at IBD diagnosis (y), median (IQR) | 22 (16-32) |
| Disease duration (y), median (IQR) | 12 (6-19) |
| Clinical remission, n(%) | |
| Yes | 119 (80) |
| Unclear | 3 (2) |
| Comorbidities, n(%) | |
| Dermatologic diseases | 19 (13) |
| Rheumatologic conditions | 18 (12) |
| Ophthalmic diseases | 3 (2) |
| Disease phenotype, n(%) | |
| Luminal | 109 (74) |
| Luminal and perianal | 39 (26) |
| Luminal surgeries, n(%) | |
| ≥ 1 | 36 (24) |
| Current medication usage, n(%) | |
| Infliximab | 96 (65) |
| Mesalazin | 3 (2) |
| Methotrexate | 6 (4) |
| Oral corticosteroids | 9 (6) |
| Thiopurines | 21 (15) |
| Vedolizumab | 53 (35) |
| Other | 7 (5) |
| Previous medication usage, n (%) | |
| Adalimumab | 37 (25) |
| Golimumab | 4 (3) |
| Inflximab | 27 (18) |
| Mesalazin | 66 (45) |
| Methotrexate | 32 (22) |
| Oral corticosteroids | 118 (80) |
| Thiopurines | 116 (78) |
| Tofacitinib | 2 (1) |
| Ustekinumab | 9 (6) |
| Vedolizumab | 9 (6) |
| Other | 36 (24) |
Table 1. Baseline characteristics.
Questionnaire results
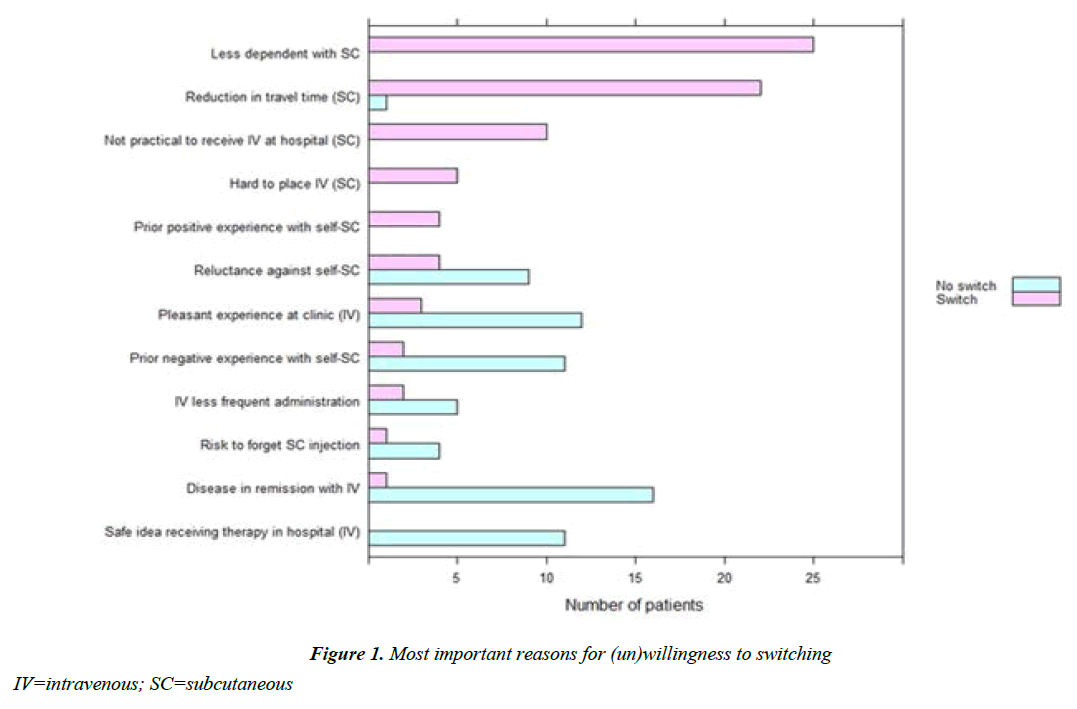
Patients reported a median satisfactory score of 8 (IQI 8-10) for the current therapy. Forty-five percent indicated to not experience difficulties regarding IV administration, 36% stated that hospital visits and travel-time were most burdensome, and 8% the drug administration time. Seventy-nine out of 148 respondents (53%) were willing to switch to SC injections. From 119 out of 148 patients who were in clinical remission at baseline, 53% were willing to switch. From the 26 out of 148 patients who were not in remission, 58% were willing to switch. The main reasons for willingness to switch to SC injections included less dependency on hospital-based care, and travel time. Patients unwilling to switch reported “disease is in remission with current IV therapy” as the main reason for preferring IV therapy. Of the patients treated with IV therapy <1 year, 65% was in clinical remission, compared to 83% in patients with IV therapy >1 year. Combined reluctance to use SC injections or prior negative experiences with SC injections also represented an important reason not to switch (Figure 1). Fifty percent of patients with CD were willing to switch, compared to 67% of patients with UC (or IBD-unclassified). Of the IFX and VDZ users, respectively, 57% and 45%, were willing to switch. The outcomes of the questionnaire are summarized in Table 2.
| Characteristics | Patients (N=148) |
|---|---|
| Willing to switch to SC, n (%) | |
| Yes | 79 (53) |
| No | 69 (47) |
| Duration IV therapy (y), n(%) | |
| 0-1 | 20 (13) |
| 1-2 | 22 (15) |
| 2-4 | 31 (21) |
| Satisfaction IV therapy (rate 0-10), median (IQR) | 8 (8-10) |
| Total travel time (min), n (%) | |
| <60 | 91 (61) |
| ≥60 | 57 (39) |
| Paid job, n (%) | 89 (60) |
Table 2. Questionnaire results.
In univariable analysis, receiving IV therapy <1 year was associated with willingness to switch to SC administration (odds ratio (OR) 4.13, 95% Confidence Interval (CI) 1.42- 15.01) and higher satisfaction with current IV therapy (OR 0.79 95% CI 0.62-0.98) was associated with unwillingness to switch to SC administration. Other variables were not found to be associated with willingness to switch in univariable logistic regression analyses. Only receiving IV therapy <1 year was associated with willingness to switch in multivariable analysis (OR 3.44, CI 1.10-13.19) (Table 3).
| Characteristics | Univariable analysis, OR (95% CI) | P-value | Multivariable analysis, OR (95% CI) | P-value |
|---|---|---|---|---|
| Age | 0.99 (0.97-1.01) | 0.23 | 0.99 (0.97-1.02) | 0.18 |
| IV therapy duration (<1 versus ≥1 y) | 4.13 (1.42-15.01) | 0.02 | 3.44 (1.10-13.19) | <0.05 |
| Satisfaction with current IV therapy (per step increase, scale 0-10) |
0.79 (0.62-0.98) | <0.05 | 0.85 (0.65-1.10) | 0.23 |
| Total travel time (≥ 60 versus <60 min) | 0.95 (0.49-1.85) | 0.89 | 0.99 (0.50-1.98) | 0.98 |
| Disease in clinical remission | 0.81 (0.34-1.89) | 0.63 | 1.19 (0.44-3.18) | 0.75 |
| Disease duration < 10 versus ≥ 10 y |
0.99 (0.96-1.02) | 0.45 | 1.01 (0.97-1.05) | 0.64 |
| Prior biological use | 0.81 (0.41-1.60) | 0.55 | 0.87 (0.42-1.81) | 0.72 |
Table 3. Predictors for willingness to switch to subcutaneous injections.
Discussion
Here, we explored patient preferences regarding their willingness to switch from IV to SC biological therapy. Fifty-three percent of patients with IBD, most of whom were in clinical remission, were willing to switch to SC therapy. Predictors for willingness to switch to a SC formulation in univariable analysis included lower treatment satisfaction with current IV therapy and receiving IV therapy <1 year (the latter also based on multivariable analysis). The two most important reasons to switch to SC injections included less dependency on hospital care and travel time. The main reasons not to switch to SC formulations were current remission and, combined, reluctance to use SC injections or prior negative experiences with SC injections.
Our willingness to switch rate of 53% is in line with recent studies that reported percentages ranging from 50-69% [6,8,9]. Overton et al. (2021) performed a systematic review on reasons to switch from SC to IV therapy in patients with chronic immune system disorders, documenting reasons comparable to our findings [8]. In this study, respondents who preferred SC over IV therapy, reported the following motivations: not needing to travel to hospital, convenience of therapy at home, greater autonomy, and easier to combine with work. Patients preferring IV treatment, named social contact, dislike of SC injections, preferring a less frequent treatment, and of presence medical staff.
Strengths of the present study include the high response rate. Although it should be noted that a small group of patients declined study participation up-front potentially affecting our results. The most important limitation is the use of a nonvalidated questionnaire in the present study. Nevertheless, the outcomes of our questionnaire are in accordance with other studies [8,9]. Furthermore, some results from this study might be difficult to extrapolate to other countries, because of considerable differences in health care and social security systems, as well as journey distances to hospital.
Conclusion
Fifty-three percent of IBD patients on IV IFX or VDZ are willing to switch to a SC formulation. The most common reason for staying on IV medication was disease in remission with current IV therapy and, combined, reluctance to use SC injections or prior negative experiences with SC injections. Predictors for willingness to switch to SC administration included lower treatment satisfaction with current IV therapy and receiving IV therapy <1 year. These results underscore that a considerable number of patients with IBD on IV therapy will accept a switch to the SC route. This will reduce inhospital resource requirements and might reduce costs.
Author Contributions
L.O. Data collection, data analysis, drafting of the manuscript
A.M.W. patient recruitment, data analysis, drafting of the manuscript
B.O. Study design, drafting of the manuscript
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Disclosures
BO reports grants from MSD, Abbvie, Takeda, Cablon, Ferring, Falk, and Pfizer. The remaining authors have no conflicts of interest to declare.
Funding Statement
Unrestricted research support for this this study was provided by Celltrion, Inc. (Incheon, South Korea) and Takeda Pharmaceuticals (Tokyo, Japan).
Acknowledgements
We thank M.G. de Schipper, L. Noteboom, and A.C. Muijsertvan Os for their help conducting this study.
References
- Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. ACG. 2009;104(3):760-7.
- Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4(5):341-53.
- Côté-Daigneault J, Bouin M, Lahaie R, et al. Biologics in inflammatory bowel disease: what are the data?. United European Gastroenterol J. 2015;3(5):419-28.
- Schreiber S, Ben-Horin S, Leszczyszyn J, et al. Randomized controlled trial: subcutaneous vs intravenous infliximab CT-P13 maintenance in inflammatory bowel disease. Gastroenterol. 2021;160(7):2340-53.
- Sandborn WJ, Baert F, Danese S, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterol. 2020;158(3):562-72.
- Ihle-Hansen H, Berge T, Tveita A, et al. COVID-19: symptoms, course of illness and use of clinical scoring systems for the first 42 patients admitted to a Norwegian local hospital. Tidsskrift for Den norske legeforening. 2020.
- McGoran J, Wilson A, McErlain S, et al. Initiation of subcutaneous infliximab (Remsima) therapy for the treatment of inflammatory bowel disease during the COVID-19 pandemic. Frontline Gastroenterol. 2022;13(1):89-90.
- Overton PM, Shalet N, Somers F, et al. Patient preferences for subcutaneous versus intravenous administration of treatment for chronic immune system disorders: A systematic review. Patient Prefer Adherence. 2021;15:811.
- Asnong K, Pouillon L, Bossuyt P. Is Also the Patient Ready for Switching from Intravenous to Subcutaneous Biologics?. JCC. 2022;16(3):515-6.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref