Research Article - Biomedical Research (2017) Volume 28, Issue 2
Palmitic acid induced vascular endothelial cell apoptosis contributes to the high severity of coronary artery disease in the Uygur population
Hailong Jiang1*, Yihua Zeng1, Li Bai1, Guanghui Wang2, Aimin Hu1
1Department of Emergency, General Hospital of Air Force, Beijing 100036, China
2Aviation Medicine Identification and Training Center of Air Force, Lintong 710600, China
- *Corresponding Author:
- Hailong Jiang
Department of Emergency
General Hospital of Air Force
Beijing 100036, China
Accepted date: July 27, 2016
Abstract
Coronary artery disease (CAD) is a common heart disease in Chinese adults with high mortality rate. Compared to the Han patients, the disease degree is often severer in the Uygur patients with coronary artery disease. In this study, using metabonomic analysis, we found that there was elevated level of saturated fatty acid in the serum from the Uygur patients. Human umbilical vein endothelial cells cultured with 400 μmol/l palmitic acid for 48 hours led to a 69.4% cell apoptosis. The expression level and activity of caspase3 were increased after palmitic acid treatment. In addition, palmitic acid promoted the secretion of TNF-α, as well as elevated intracellular reactive oxygen species accumulation. Western blot analysis revealed that p-p38 level was raised during this process. Blocking p38 signal by inhibitor significantly attenuated the pro-apoptosis effects of palmitic acid, indicating a pivotal role of p38 pathway in this progression. These results provide some evidences for understanding the differences of disease degree between the Uygur and Han CAD patients.
Keywords
Coronary artery disease, Palmitic acid, Uygur population, p38 MAPK signal.
Introduction
Coronary Artery Disease (CAD), a world-wide, high risk heart disease, is the leading cause of death in Chinese adults with cardiac disease. Atherosclerosis is considered to be the primary pathological changes in CAD, which also acts as a causing factor on the other hand. Vascular endothelial cells (VEC) play important roles in the pathogenesis of coronary artery disease. The intact structures and functions of ECs are indispensable for preventing the occurrence of atherosclerosis [1], and the impairment of EC functions might be the initial step of the disease [2]. The pathogenic factors of CAD, such as old age, hyperglycemia and hyperlipidemia, could reduce the number of circulating ECs and attenuate their adhesion, migration and regeneration abilities [3]. In fact, the disorder of VEC functions in CAD has been emphasized for a long time. Meanwhile, it also provides a therapeutic target for coronary artery disease [4].
Abnormal lipid metabolism is a predominant factor in the pathogenesis of atherosclerosis. Palmitic acid (PA) is a member of the free fatty acids (FFA) family, whose disorder is observed in insulin-resistant states including diabetes, obesity, and dyslipidemias. High concentration FFA impedes the adhesion and proliferation of endothelial cells, which might contribute to the pathogenesis of atherosclerosis [5,6]. Moreover, treatment with palmitic acid induces the apoptosis of many cell types, including cardiomyocytes [7,8], suggesting a potential role of PA in coronary artery disease.
Metabonomic analysis, a powerful tool for revealing the relationship between metabolites and biological phenomena, has been widely used in pharmaceutical research and development. Variations of metabolites are able to reflect the subtle changes of body inner environment. Magnetic Resonance (MR) is the most frequently utilized analysis method in metabonomics. Using metabonomic analysis based on HMR, Brindle et al. established a fast, non-invasive diagnosis method for cardiac coronary disease [9]. In this study, we used NMR to analyze the difference of metabolites in the serum from the Han and Uygur CAD patients, revealing an increased palmitic acid level in the Uygur individuals. It might be correlated with higher pathologic degree in the Uygur patients. Moreover, treatment of endothelial cells with palmitic acid promoted apoptosis through activating MAPK pathway. These results are instrumental in exploring the mechanism of high susceptibility for coronary artery disease in the Uygur population.
Materials and Methods
NMR analysis of metabolites in serum
Blood was collected from 13 Uygur and 11 Han CAD patients in the Urumqi General Hospital of PLA. The serum was prepared by centrifugation and subjected to NMR analysis, as described previously [9]. Briefly, H-NMR spectra of the samples was measured with the pulse sequence: -RD-90°-G1-180°-G1-90°-T-90°-G1-180°-G1-90°-π-90°-ACQ. The acquired data were processed using PLS-DA to analyze the differences between the Uygur and Han patients.
Cells and reagents
Human Umbilical Vein Endothelial Cell (HUVEC) was obtained from American Type Culture Collection, and cultured in M199 culture medium (Gibco) supplemented with 20% FBS. Palmitic acid was purchased from Sigma-Aldrich. The anti-Caspase 3, anti-p38, anti-p-p38, anti-JNK, anti-p-JNK, anti-ERK1/2, anti-p-ERK1/2, anti-Actin antibodies, as well as NADPH oxidase inhibitor Apocynin were purchased from Santa-Cruz. Caspase 3 Activity Assay Kit, Annexin V-FITC Apoptosis Detection Kit and dichlorofluorescein diacetate (DCFH-DA) detection kit were purchased from Beyotime Biotechnolgy. TNF-α and IL6 Elisa Kits were obtained from eBioscience. SB203580, PD98059, SP600125 were obtained from Stressgen Bioregent.
HUVEC apoptosis assay
Firstly, HUVECs were cultured in M199 medium supplemented with 2% FBS for 12 hours, then these cells were treated with palmitic acid for an indicated time period. Treated cells were stained with Annexin V-FITC and subjected to FACS to measure the apoptosis cell ratio. The proteins were detected by Western blot. The caspase 3 activities were quantified using Caspase 3 Activity Assay Kit, following the instructions of manufacturer.
TNF-α and IL6 measurement
The supernatants of HUVECs were collected and subjected to ELISA analysis using TNF-α and IL6 Elisa Kits, following the manufacturer instructions.
Treatment of HUVECs with signal pathway inhibitors
HUVECs were cultured in M199 medium supplemented with 2% FBS for 12 hours, then 10 μmol/l SB203580 (p38 inhibitor) or 50 μmol/l PD98059 (ERK inhibitor) or 50 μmol/l SP600125 (Jnk inhibitor) were added into cell culture. 1 hour later, 400 μmol/l palmitic acid was supplemented into medium and the cells were treated for 47 additional hours. The supernatants and cells were separately collected for further analysis.
Reactive oxygen species (ROS) assay
HUVECs were treated with different concentration of Apocynin for 1 hour. After that, cells were stimulated with 400 μmol/l palmitic acid for an additional 47 hours. The treated cells were stained with DCFH-DA to detect the intracellular reactive oxygen species, following the manufacturer instructions. Rosup, a compound mixture stimulating reactive oxygen, was provided in the kit. Rosup treated cells were positive control and the untreated cells were negative control.
Statistics
All data was analyzed using Graphpad Prism software. The significance of difference was measured by student’ t test. p<0.05 was considered to be significant.
Results
Elevated saturated fatty acid was detected in the serum from the Uygur CAD patients
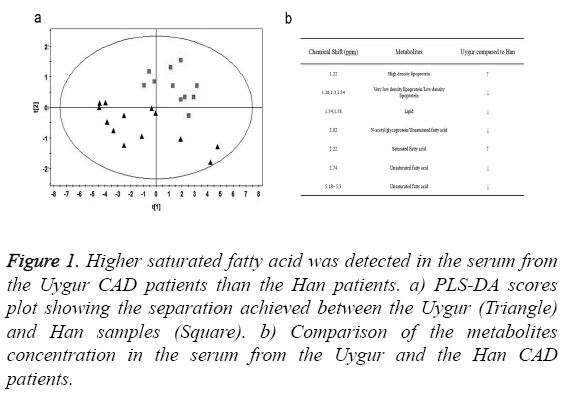
We collected serum from 13 Uygur and 11 Han CAD patients and measured metabolites concentration using NMR. PLS-DA analysis was performed to make a clear separation between the Uygur and Han samples (Figure 1a). Compared to the Han patients, there were higher concentration of saturated fatty acid and high density lipoprotein, as well as lower concentration of unsaturated fatty acid and low density lipoprotein in the serum from the Uygur patients (Figure 1b). It indicated that high level saturated fatty acid might be a factor attributing to the severer CAD conditions in the Uygur patients.
Figure 1: Higher saturated fatty acid was detected in the serum from the Uygur CAD patients than the Han patients. a) PLS-DA scores plot showing the separation achieved between the Uygur (Triangle) and Han samples (Square). b) Comparison of the metabolites concentration in the serum from the Uygur and the Han CAD patients.
Palmitic acid induced the apoptosis of human umbilical vein endothelial cells
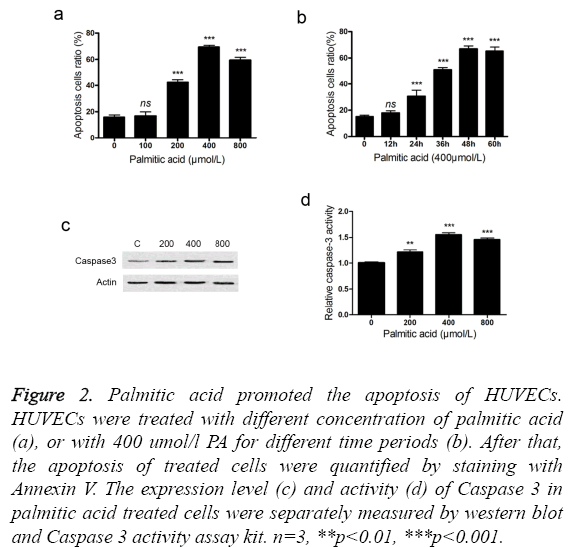
We use palmitic acid, a main type of saturated fatty acid, to explore the roles of SFA in the pathogenesis of CAD. Treatment with palmitic acid induced the apoptosis of HUVECs in a dose and time related manner (Figures 2a and 2b). In the tested conditions, treatment with 400 umol/l palmitic acid for 48 hours led to the highest apoptosis ratio. It should be noted that low concentration (100 umol/l) or short treatment time (12 hours) exhibited little effect on apoptosis induction. In addition, treatment with PA increased the expression level of Caspase 3 (Figure 2c). An increment of Caspase 3 activity was also detected in the PA treated HUVECs (Figure 2d). It suggested that PA induced the apoptosis of HUVECs through activating caspase pathway.
Figure 2: Palmitic acid promoted the apoptosis of HUVECs. HUVECs were treated with different concentration of palmitic acid (a), or with 400 umol/l PA for different time periods (b). After that, the apoptosis of treated cells were quantified by staining with Annexin V. The expression level (c) and activity (d) of Caspase 3 in palmitic acid treated cells were separately measured by western blot and Caspase 3 activity assay kit. n=3, **p<0.01, ***p<0.001.
Palmitic acid enhanced the secretion of TNF-α in HUVECs
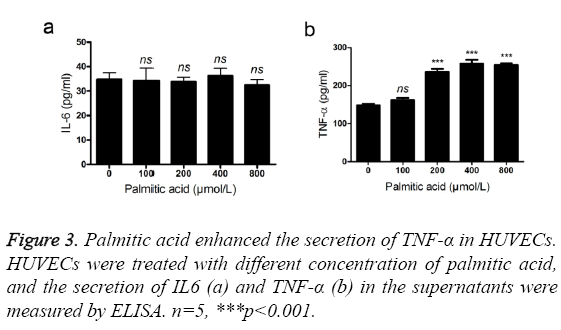
TNF-α and IL6 are two important inflammatory cytokines in atheroselerosis [10,11]. Therefore, we evaluated the effects of PA on the secretion of TNF-α and IL6. High concentration of PA (>200 umol/l) promoted the secretion of TNF-α in HUVECs (Figure 3a). However, the expression of IL6 was not affected after addition of PA (Figure 3b). TNF-α, not IL6, is likely to be an effector cytokine of palmitic acid.
Palmitic acid promoted the accumulation of intracellular reactive oxygen species
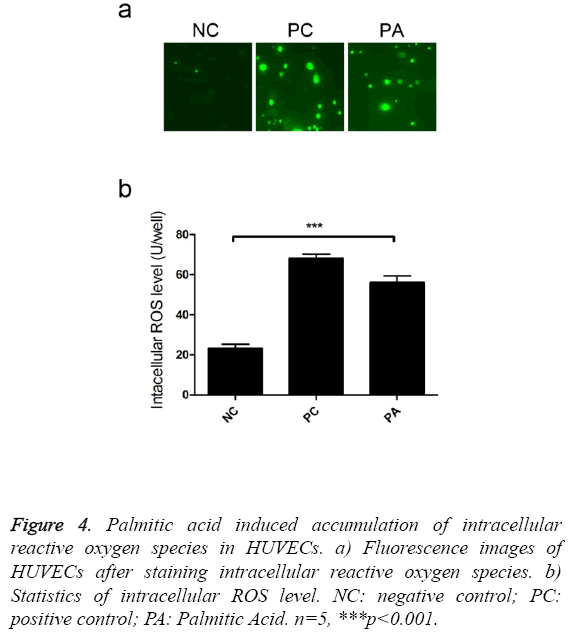
Reactive oxygen species could impair endothelial cell functions [12], thus we investigate the effects of palmitic acid on ROS production. Intracellular staining revealed that addition of palmitic greatly enhanced the accumulation of reactive oxygen species (Figure 4a). There were 2.4 fold ROS in PA treated cells than control cells (Figure 4b), which was cytotoxic to host cells.
Figure 4: Palmitic acid induced accumulation of intracellular reactive oxygen species in HUVECs. a) Fluorescence images of HUVECs after staining intracellular reactive oxygen species. b) Statistics of intracellular ROS level. NC: negative control; PC: positive control; PA: Palmitic Acid. n=5, ***p<0.001.
p38 MAPK signal pathway and NADPH oxidase participated in palmitic acid induced cell apoptosis
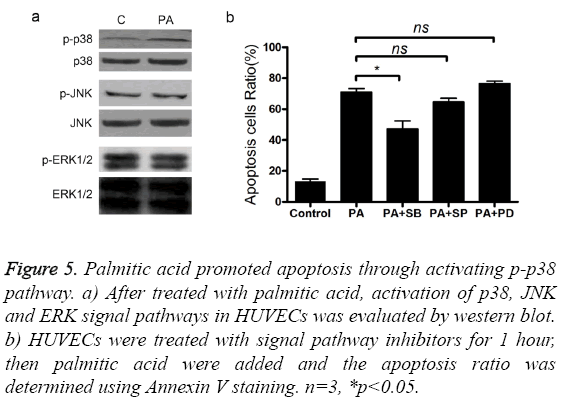
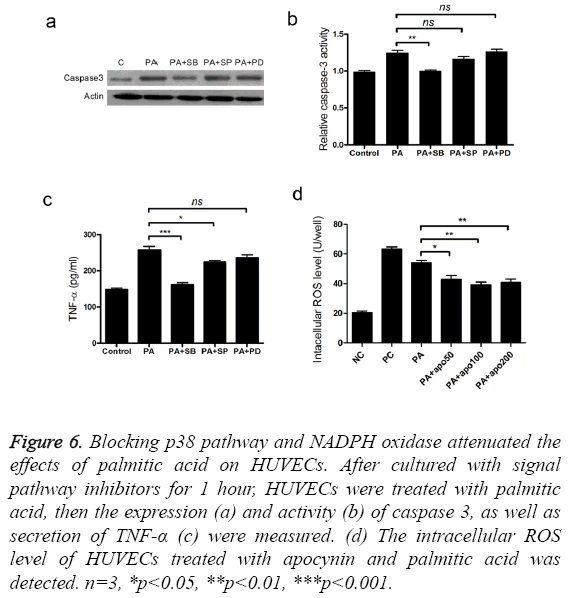
To find out the signal pathways mediating the cytotoxic activity of palmitic acid, we measured the changes of p38, ERK and JNK protein after treatment with palmitic acid. The level of p38, ERK, p-ERK, JNK and p-JNK was not altered by the addition palmitic acid (Figure 5a). However, treatment with PA increased the p-p38 protein level in HUVECs (Figure 5a). Next, three inhibitors, including p38 inhibitor SB203580, ERK inhibitor PD98059 and JNK inhibitor SP600125 were separately added to cultured cells and the effects were measured. Coincided with western blot results, only p38 inhibitor SB203580 attenuated cell apoptosis caused by palmitic acid (Figure 5b). Furthermore, addition of SB203580 impeded PA induced elevation of caspase3 protein level and activity (Figures 6a and 6b). As well, the secretion of TNF-α was also inhibited by SB203580 (Figure 6c). These results indicated that the functions of palmitic acid on endothelial cells were mediated by p38 MAPK pathway.
Figure 5: Palmitic acid promoted apoptosis through activating p-p38 pathway. a) After treated with palmitic acid, activation of p38, JNK and ERK signal pathways in HUVECs was evaluated by western blot. b) HUVECs were treated with signal pathway inhibitors for 1 hour, then palmitic acid were added and the apoptosis ratio was determined using Annexin V staining. n=3, *p<0.05.
Figure 6: Blocking p38 pathway and NADPH oxidase attenuated the effects of palmitic acid on HUVECs. After cultured with signal pathway inhibitors for 1 hour, HUVECs were treated with palmitic acid, then the expression (a) and activity (b) of caspase 3, as well as secretion of TNF-α (c) were measured. (d) The intracellular ROS level of HUVECs treated with apocynin and palmitic acid was detected. n=3, *p<0.05, **p<0.01, ***p<0.001.
We have found palmitic acid stimulated the accumulation of ROS, thus apocynin, a NADPH oxidase inhibitor, was used to reveal the effective pathway. Treatment with apocynin alleviated the excessive accumulation of ROS (Figure 6d), which was beneficial to cell viability. It indicated that NADPH oxidase was involved in the function of palmitic acid.
Discussion
Metabonomic analysis provides a useful tool to monitor the physiological status of individuals. The products of cellular metabolism directly enter into the body fluid, making it possible to reveal pathological state via measuring metabolites in blood and urine. Using metabonomic analysis, Yang et al. accurately classified diabetic patients from healthy persons, providing a new method for diabetic diagnosis [13]. It has been demonstrated that the metabolic changes of fatty acid was long before the emergence of type II diabetic symptom [14]. Palmitic acid, linolenic acid and hydroxybutyric acid in plasma are found to be closely related with the occurrence of diabetic [15]. Using this method, Rosiglitazone was demonstrated to alter the metabolism of polyunsaturated fatty acid [16]. In our study, we found some differences of metabolites in serum between the Han and Uygur CAD patients, using metabonomic analysis. Saturated fatty acid and high density lipoprotein are significantly increased in the Uygur patients, as well as decreased unsaturated fatty acid, low density lipoprotein and lipid. As metabolites in the bodies could be influenced by many factors, such as races [17], ages [18], gender [19], environment [20], diet [21], it can be envisaged that the different lifestyles between the Han and Uygur peoples in Xinjiang province lead to the metabolites variations, ultimately making the Uygur CAD patients with more severe disease degrees.
The impairment of vascular endothelial cells is of key importance in the pathogenesis of atherosclerosis. The endothelial cells derived from bone marrow gradually exhausted with age, making old people more susceptible to atherosclerotic diseases [5]. In a study, injecting bone marrow cells to mice with high fat diet successfully ameliorated atherosclerotic syndrome [6]. On the other hand, as an important factor in type II diabetes [22], free fatty acid is also proved to be closely associated with atherosclerosis [23,24]. Treatment with high glucose impaired the adhesion and proliferation of endothelial cells, as well as promoted their senescence. However, the influences of fatty acid metabolism dysfunction in diabetes have not been well described. In our study, we found that palmitic acid, a predominant subtype of free fatty acid, promoted the apoptosis of ECs. It implied that the elevated saturate fatty acid in the serum of the Uygur patients promoted the disease progression through injuring vascular endothelial cells. Notably, palmitic acid stimulated HUVECs to secret TNF-α, a pro-inflammatory cytokine. Atherosclerosis is considered to be an inflammatory disease [10], thus it represents another pathway of free fatty acid to promote disease. Treatment of ECs with TNF-α led to an increased expression of VCAM-1 and ICAM-1, as well as a higher apoptosis ratio [25]. Like TNF-α, IL6 is a frequently observed, pro-inflammatory factor in the process of artery endothelium injury. But in our results, palmitic acid did not enhance the expression of IL6, indicating other potential factors promoting inflammation in this disease. We found that intracellular ROS was increased after addition of palmitic acid, which could directly promote the senescence of host cells. Indeed, ROS is able to impair endothelial progenitor cell function [26]. Taken together, it can be imaged that palmitic acid induces endothelial cell apoptosis via increasing the expression of TNF-α and the accumulation of intracellular ROS.
MAPK pathway is an important signal cascade in inflammatory responses, which plays a pivotal role in the apoptosis of endothelial cells. Activation of p38 and JNK pathways is able to upregulate the secretion of TNF-α and intracellular ROS level, promoting endothelial cell senescence [27,28]. Through impeding different pathways by inhibitors, we revealed that palmitic acid executed the roles via p38 MAPK pathway. Increment of p-p38 is dispensable for palmitic acid induced cell apoptosis and TNF-α secretion, because blocking it with inhibitor SB203580 abolishes these effects. Of note, some other signal pathways contribute to the outcome of PA treatment as well. Guo et al. demonstrated that PA promoting apoptosis through suppressing AKT/eNOS pathway [29]. Nevertheless, our research provides new insight into the pathogenesis of atherosclerosis in the Uygur population.
References
- Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest 2000; 106: 571-578.
- 2Libby P, Ridker PM,Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105: 1135-1143.
- Tepper OM, Galiano RD, Capla JM. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002; 106: 2781-2786.
- Gimbrone MA Jr. Vascular endothelium: an integrator of pathophysiologic stimuli in atherosclerosis. Am J Cardiol 1995; 75: 67B-70B.
- Perez FR, Pineiro V, De La Cruz LF, Casanueva FF, Casabiell X. Vascular wall: potential target for the physicochemical effects of cis-unsaturated free fatty acids. Microsc Res Tech 2003; 60: 23-29.
- Stentz FB, Kitabchi AE. Palmitic acid-induced activation of human T-lymphocytes and aortic endothelial cells with production of insulin receptors, reactive oxygen species, cytokines, and lipid peroxidation. BiochemBiophys Res Commun 2006; 346: 721-726.
- Haversen L, Danielsson KN, Fogelstrand L, Wiklund O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 2009; 202: 382-393.
- Moers A, Schrezenmeir J. Palmitic acid but not stearic acid inhibits NO-production in endothelial cells. ExpClinEndocrinol Diabetes 1997; 105: 78-80.
- Brindle JT, Antti H, Holmes E. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med 2002; 8: 1439-1444.
- Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med 1999; 340: 115-126.
- Puhakka M, Magga J, Hietakorpi S. Interleukin-6 and tumor necrosis factor alpha in relation to myocardial infarct size and collagen formation. 2003; J Card Fail 9: 325-332.
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006; 440: 944-948.
- Yang J, Xu G, Hong Q. Discrimination of Type 2 diabetic patients from healthy controls by using metabonomics method based on their serum fatty acid profiles. J Chromatogr B AnalytTechnol Biomed Life Sci 2004; 813: 53-58.
- Zhao X, Fritsche J, Wang J. Metabonomic fingerprints of fasting plasma and spot urine reveal human pre-diabetic metabolic traits. Metabolomics 2010; 6: 362-374.
- Li X, Xu Z, Lu X. Comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry for metabonomics: Biomarker discovery for diabetes mellitus. Anal ChimActa 2009; 633: 257-262.
- Watkins SM, German JB. Toward the implementation of metabolomic assessments of human health and nutrition. CurrOpinBiotechnol 2002; 13: 512-516.
- Lenz EM, Bright J, Wilson ID. Metabonomics, dietary influences and cultural differences: a 1H NMR-based study of urine samples obtained from healthy British and Swedish subjects. J Pharm Biomed Anal 2004; 36: 841-849.
- Wang Y, Lawler D, Larson B. Metabonomic investigations of aging and caloric restriction in a life-long dog study. J Proteome Res 2007; 6: 1846-1854.
- Kochhar S, Jacobs DM, Ramadan Z, Berruex F, Fuerholz A, Fay LB. Probing gender-specific metabolism differences in humans by nuclear magnetic resonance-based metabonomics. Anal Biochem 2006; 352: 274-281.
- Viant MR, Rosenblum ES,Tieerdema RS. NMR-based metabolomics: a powerful approach for characterizing the effects of environmental stressors on organism health. Environ SciTechnol2003; 37: 4982-4989.
- Wang Y, Holmes E, Tang H. Experimental metabonomic model of dietary variation and stress interactions. J Proteome Res 2006; 5: 1535-1542.
- McGarry JD.Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002; 51: 7-18.
- Smith SR, Wilson PW. Free fatty acids and atherosclerosis-guilty or innocent? J ClinEndocrinolMetab2006; 91: 2506-2508.
- Armstrong KA, Hiremagalur B, Haluska BA. Free fatty acids are associated with obesity, insulin resistance, and atherosclerosis in renal transplant recipients. Transplantation 2005; 80: 937-944.
- Hoffman GS, Merkel PA, Brasington RD, Lenschow DJ, Liang P. Anti-tumor necrosis factor therapy in patients with difficult to treat Takayasu arteritis. Arthritis Rheum 2004; 50: 2296-2304.
- Callaghan MJ, Ceradini DJ,Gurtner GC.Hyperglycemia-induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxid Redox Signal 2055; 7: 1476-1482.
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001; 410: 37-40.
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res 2005; 15: 11-18.
- Guo WX, Yang QD, Liu YH, Xie XY, Wang M,Niu RC.Palmitic and linoleic acids impair endothelial progenitor cells by inhibition of Akt/eNOS pathway. Arch Med Res 2008; 39: 434-442.