Research Article - Biomedical Research (2017) Volume 28, Issue 17
Paclitaxel inhibits growth and proliferation of glioblastoma through MMP-9-meidated p38/JNK signaling pathway
Dong Ruan1, Xin Li2, Aixia Li3, Baohui Liu4 and Feng Xu1*
1Department of Neurosurgery, Xianning Central Hospital, the First Affiliated Hospital of Hubei University of Science and Technology, Xianning City, PR China
2Department of Computer Science and Technology College, Hubei science and Technology University, Xianning City, PR China
3Department of Encephalopathy, Xianning Hospital of Traditional Chinese Medicine, Xianning City, PR China
4Department of Neurosurgery, Renmin Hospital, Wuhan University, Wuhan City, PR China
- *Corresponding Author:
- Feng Xu
Department of Neurosurgery
Xianning Central Hospital
The First Affiliated Hospital of Hubei University of Science and Technology, PR China
Accepted date: February 27, 2017
Abstract
Glioblastoma is the most aggressive primary brain tumors originated from the glial cells in adults, which is characterized by stronger migration and invasion. Paclitaxel is an efficient anti-glioblastoma drug for patients in clinical. However, potential molecular mechanism of paclitaxel-inhibited growth and proliferation of glioblastoma has not been well understood yet. In this study, we investigated signal pathway mediated by paclitaxel in glioblastoma cells. Our results demonstrated that paclitaxel treatment inhibited growth and proliferation of glioblastoma cell line U251. Paclitaxel administration induced apoptosis of U251 cells by up-regulation of caspase signal pathway. Mechanism analyses showed that paclitaxel addition down-regulated MMP-9 expression, which further inhibited p38/JNK signaling pathway in U251 cells. In vivo assays showed that Paclitaxel treatment could significantly inhibit glioblastoma growth and prolong survival of tumor-bearing mice. In conclusion, these results indicate Paclitaxel administration inhibits growth and proliferation of glioblastoma through MMP-9-meidated p38/JNK signaling pathway, which contributes to understanding signal pathway of paclitaxelsuppressed glioblastoma growth.
Keywords
Glioblastoma, Paclitaxel, Proliferation, MMP-9, p38/JNK
Introduction
Malignant glioma is the most common primary brain carcinoma and presents poor survival rate in the world, which is characterized by the appearance of vascular proliferation, aggressive invasion, and necrosis around human normal brain tissues [1-3]. Glioblastoma are the most common and lethal primary tumors of the central nervous system due to strong invasive and heterogeneous nature as well as resistance to multimodal treatments of anti-cancer drugs [4,5]. Glioblastoma exhibits a poor prognosis despite maximal multimodal therapy and the median survival of most patients is less than one year after application of multimodal therapies [6,7]. Therefore, understanding potential mechanism glioblastoma of growth and proliferation contributes to analyse signal pathway and explore more targets for the treatment of glioblastoma.
Paclitaxel is a tricyclic diterpene compound that loaded biodegradable poly-(DL-lactic-co-glycolic) acid (PLGA) foams with microporous matrix [8]. Paclitaxel presents better therapeutic effects for human cancer, such as ovarian cancer and breast cancer, lung cancer, colorectal cancer, melanoma, head and neck cancer, lymphoma, as well as brain tumors [9-11]. Woo et al. have paclitaxel can induce apoptotic cell death in human glioblastoma U87MG cells via regulation of apoptotic functions of p53 and c-Jun N-Terminal Kinase [JNK] [12]. In addition, human CD14+ cells loaded with paclitaxel inhibit glioblastoma cells proliferation in vitro, which suggest that Paclitaxel could be used to delivery anti-cancer immunotherapy drugs to kill glioblastoma cells [13]. Furthermore, Merighi et al. have suggested that paclitaxelinduced apoptosis through regulation of the extracellular signal-regulated kinase 1/2 activities, which is able to modulate Bad phosphorylation [11]. However, MMP-9-meidated p38/JNK signaling pathway in growth and proliferation mediated by Paclitaxel has not been investigated in the progression of glioblastoma.
In the current study, we analysed the potential mechanism of glioblastoma cells growth and proliferation during paclitaxel treatment. This study assumed that paclitaxel inhibited growth and metastasis of glioblastoma cells through regulation of MMP-9-meidated p38/JNK signaling pathway. Furthermore, we provide strong evidences that Paclitaxel can be regarded as potential anti-cancer drug for the treatment of glioblastoma via inhibition of growth and proliferation.
Materials and Methods
Ethic statement
This analysis was performed by the recommendations in the Guide for the Care and Use of Laboratory Animals. All animals’ protocols and animals were performed in accordance with National Institutes of Health and approved by Committee on the Ethics of Animal Experiments Defence Research.
Cells culture
U251 and U87MG cells were purchased from American Type Culture Collection (ATCC). All cells were cultured in DMEM (Sigma-Aldrich) medium (Gibco, CA, USA) supplemented with 10% Fetal Bovine Serum (FBS) (Invitrogen, CA, USA). All cells were cultured in a 37°C humidified atmosphere of 5 % CO2.
MTT assay
U251 and U87MG cells were incubated with Paclitaxel (5, 10, 15 or 20 mg/ml) and or MMP inhibitor (ab142180, China) in 96 well plates for 24, 48 and 72 h in triplicate for each condition with PBS as control. After incubation, 20 μl of MTT (5 mg/ml) in PBS solution was added to each well, the plate was further incubated for 4 hours. Most of the medium was removed and 100 μl of DMSO was added into the wells to solubilize the crystals. The OD was measured by a BIO-RAD (ELISA) reader at wavelength of 450 nm.
MMP-9 overexpression
U251 and U87MG cells were cultured until 90% confluence and the media was then removed. U251 and U87MG cells were washed and transfected by lentivirus-MMP-9 (pMMP-9) using lipfectamine 2000 (Sigma-Aldrich). Protein expression levels of purpose protein were analysed in MMP-9-overexpressed U251 and U87MG cells.
Real-Tome quantitative PCR (RT-qPCR)
Total RNA was extracted from U251 and U87MG and the identified RNA was applied to the cDNA synthesis by reverse transcription PCR. One-tenth of the cDNA was used to fluorescent quantitative RT-PCR by using iQ SYBR Green System. Relative multiples of change in mRNA expression was calculated by 2-ΔΔCt. The results are expressed as the n-fold difference relative to normal β-actin control.
Apoptosis assay
U251 and U87MG cells were incubated with paclitaxel (15 mg/ml) for 48 h. After incubation, all tumor cells were trypsinized and collected. Subsequently, U251 and U87MG cells were washed in cold PBS and adjusted to 1 × 106 cells/ml with PBS, labeled with annexin V-FITC and PI (Annexin VFITC Kit, BD), and analysed with a FACScan flow cytometer (BD).
Western blotting
U251 and U87MG cells and tumors were homogenized in lysate buffer containing protease-inhibitor and were centrifuged at 8000 rpm/min at 4°C for 10 min. The purpose protein expression levels were incubated with rabbit antihuman primary antibodies anti-MMP-9 (1:500; Cell Signaling, Danvers, MA, USA), anti-p38 (1:500; Cell Signaling, Danvers, MA, USA), anti-JNK (1:1000; Cell Signaling, Danvers, MA, USA), anti-phospho-JNK (Thr183/Tyr185) (1:1000; Cell Signaling), and anti-actin (1:5000; Millipore, Billerica, MA, USA). The following were used: HRP-labeled IgG (Abcam, Shanghai, China) for 1 h at 37°C. All proteins were visualized by using chemi-luminescence detection system.
Animal study
Specific pathogen-free male Balb/c mice were purchased from Slack co., LTD (Shanghai, China). Nude mice were subcutaneously implanted U251 (106) or U87MG (106) into experimental mice. Mice were divided into two groups (n=30). Treatments were initiated on day 6 after tumor implantation (diameter: 5-6 mm). Tumor-bearing mice were intravenously injected paclitaxel (10 mg/kg) with PBS as control. The treatment was continued 9 times once time a day. The tumor volumes were calculated to access the efficacy of Paclitaxel for tumor inhibition according to previous study [14].
Immunohistochemistry
Tumors from xenografted mice were fixed by using formaldehyde (10%) followed with embed in paraffin. Antigen retrieval was performed in tumor sections and the sections were incubated with rabbit anti-mouse primary antibodies anti- MMP-9 (1:500; Cell Signaling, Danvers, MA, USA), anti-p38 (1:500; Cell Signaling, Danvers, MA, USA), anti-JNK (1:1000; Cell Signaling, Danvers, MA, USA). Tumor tissues were washed with PBS three times and incubated with biotinylated secondary antibodies anti-rabbit IgG (all 1:200; Pierce Biotechnology, Rockford, IL, USA). Biotin-peroxidase signals were detected using 0.5 mg/mL 3’3’-Diaminobenzidine (DAB)/0.003% H2O2 (Dako, Carpinteria, CA, USA) as a substrate. Results were recorded using a microscope (BX51; Olympus, Tokyo, Japan).
Statistical analysis
All date were expressed as mean ± SD of triplicate dependent experiments and analysed by using student t tests or one-way ANOVA (Tukey HSD test). All data were analysed using SPSS Statistics 19.0 and Graphpad Prism version 5.0 with the help of Microsoft Excel. *P<0.05 and **P<0.01 were considered statistical differences.
Results
Paclitaxel inhibits growth and proliferation of glioblastoma cells in vitro
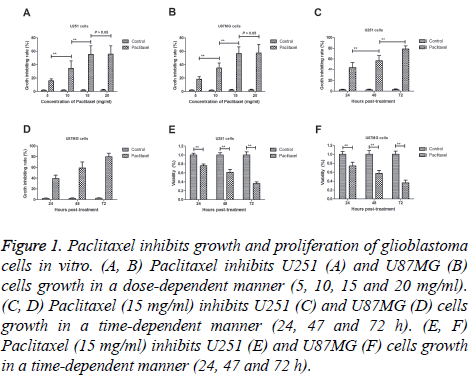
Paclitaxel exhibits anti-cancer efficacy for the treatment of glioblastoma. Results demonstrated that Paclitaxel inhibited glioblastoma cells growth in a dose-dependent manner (5, 10, 15 and 20 mg/ml; Figures 1A and 1B). Paclitaxel reached maximum inhibition for U251 and U87MG cells when concentration arrived at 15 mg/ml. We observed that paclitaxel (15 mg/ml) inhibited U251 and U87MG cells growth in a timedependent manner (24, 47 and 72 h; Figures 1C and 1D). Results also demonstrated that paclitaxel administration significantly inhibited proliferation of U251 and U87MG cells (Figures 1E and 1F). These results suggest that paclitaxel can inhibit growth and proliferation of glioblastoma cells in vitro.
Figure 1: Paclitaxel inhibits growth and proliferation of glioblastoma cells in vitro. (A, B) Paclitaxel inhibits U251 (A) and U87MG (B) cells growth in a dose-dependent manner (5, 10, 15 and 20 mg/ml). (C, D) Paclitaxel (15 mg/ml) inhibits U251 (C) and U87MG (D) cells growth in a time-dependent manner (24, 47 and 72 h). (E, F) Paclitaxel (15 mg/ml) inhibits U251 (E) and U87MG (F) cells growth in a time-dependent manner (24, 47 and 72 h).
Paclitaxel induced apoptosis of glioblastoma cells in vitro
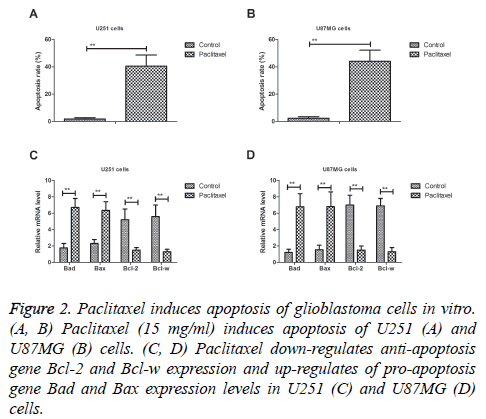
We analysed the efficacy of Paclitaxel on inducing apoptosis of glioblastoma cells. As shown in Figures 2A and 2B, Paclitaxel (15 mg/ml) significantly induced apoptosis of U251 and U87MG cells. Western blot demonstrated that showed that Paclitaxel treatment down-regulated anti-apoptosis gene Bcl-2 and Bcl-w expression and up-regulated of pro-apoptosis gene Bad and Bax expression levels in U251 and U87MG cells in vitro (Figures 2C and 2D). These results suggest that paclitaxel can significantly induce apoptosis of glioblastoma cells in vitro.
Figure 2: Paclitaxel induces apoptosis of glioblastoma cells in vitro. (A, B) Paclitaxel (15 mg/ml) induces apoptosis of U251 (A) and U87MG (B) cells. (C, D) Paclitaxel down-regulates anti-apoptosis gene Bcl-2 and Bcl-w expression and up-regulates of pro-apoptosis gene Bad and Bax expression levels in U251 (C) and U87MG (D) cells.
Paclitaxel inhibits growth and proliferation of glioblastoma through MMP-9-meidated p38/JNK signaling pathway
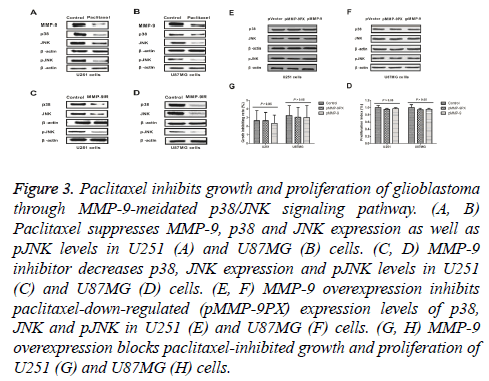
As shown in Figures 3A and 3B, paclitaxel suppressed MMP-9, p38 and JNK expression as well as JNK phosphorylation levels in U251 and U87MG cells. We observed that MMP-9 inhibitor could decrease p38, JNK expression and JNK phosphorylation (pJNK) levels in U251 and U87MG cells (Figures 3C and 3D). Results also showed that MMP-9 overexpression inhibited paclitaxel-downregulated (pMMP-9PX) expression levels of p38, JNK and pJNK in U251 and U87MG cells (Figures 3E and 3F). Importantly, paclitaxel inhibited growth and proliferation of U251 and U87MG cells was also abolished by MMP-9 overexpression (Figures 3G and 3H). These results suggest that paclitaxel can inhibit growth and proliferation of glioblastoma through MMP-9-meidated p38/JNK signaling pathway.
Figure 3: Paclitaxel inhibits growth and proliferation of glioblastoma through MMP-9-meidated p38/JNK signaling pathway. (A, B) Paclitaxel suppresses MMP-9, p38 and JNK expression as well as pJNK levels in U251 (A) and U87MG (B) cells. (C, D) MMP-9 inhibitor decreases p38, JNK expression and pJNK levels in U251 (C) and U87MG (D) cells. (E, F) MMP-9 overexpression inhibits paclitaxel-down-regulated (pMMP-9PX) expression levels of p38, JNK and pJNK in U251 (E) and U87MG (F) cells. (G, H) MMP-9 overexpression blocks paclitaxel-inhibited growth and proliferation of U251 (G) and U87MG (H) cells.
Paclitaxel inhibits glioblastoma tumor growth in tumor-bearing mice
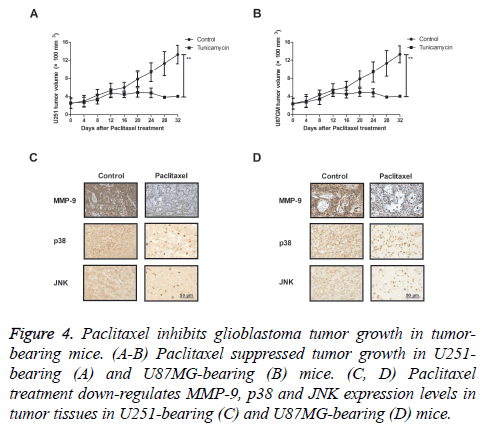
We further investigated in vivo efficacy of paclitaxel in tumorbearing mice. As shown in Figures 4A and 4B, paclitaxel suppressed tumor growth in U251- and U87MG-bearing mice. Results also showed that MMP-9, p38 and JNK expression levels were significantly down-regulated in tumor tissues after treatment with Paclitaxel (Figures 4C and 4D). These results indicate that Paclitaxel could inhibit glioblastoma tumor growth in tumor-bearing mice.
Figure 4: Paclitaxel inhibits glioblastoma tumor growth in tumorbearing mice. (A-B) Paclitaxel suppressed tumor growth in U251- bearing (A) and U87MG-bearing (B) mice. (C, D) Paclitaxel treatment down-regulates MMP-9, p38 and JNK expression levels in tumor tissues in U251-bearing (C) and U87MG-bearing (D) mice.
Discussion
Glioblastoma exhibits a poor prognosis despite maximal multimodal therapy. Patients with an advanced glioblastoma are common accompanied with symptom of seizures and/or stroke which increased the difficulty and risk of clinical treatment [15]. Statistical review and meat-analysis has revealed that glioblastoma accounted for approximately 75% in all malignant tumors related the brain [16]. Evidences have indicated that paclitaxel presents anti-cancer potential for human malignant tumors through inducing apoptosis and inhibiting tumor cells growth and proliferation [17-19]. In this study, we investigated inhibitory effects of paclitaxel on glioblastoma cells. Findings indicate that paclitaxel markedly suppresses growth and proliferation as well promotes apoptosis of U251 and U87MG both in vitro and in vivo. Notably, we found that paclitaxel can inhibit growth and proliferation of glioblastoma through MMP-9-meidated p38/JNK signaling pathway.
Apoptosis sensitivity is crucial for the inhibition of glioblastoma growth [20,21]. Researches have showed that pro-apoptosis of Bad and Bax down-regulation enhance apoptotic resistance of glioblastoma cells [11,22]. Previous reports also suggest that anti-apoptosis of Bcl-2 expression was down-regulated in glioblastoma cells that potential impact on the resistance of glioblastoma to temozolomide [23,24]. Additionally, Bcl-w enhances mesenchymal changes and invasiveness of glioblastoma cells by inducing nuclear accumulation of beta-catenin, which promotes growth and invasive potentials induced by Bcl-w down-regulation in glioblastoma multiforme [25,26]. In this study, our investigations revealed that paclitaxel treatment can significantly induce apoptosis of glioblastoma cells via inhibition of Bcl-2 and Bcl-w expression, up-regulation of Bad and Bax expression levels in vitro. Involvement of apoptotic signaling pathways induced by paclitaxel contributes to inhibition of growth and proliferation of glioblastoma.
Currently, MMP-9 mediates signal pathways have interested oncologists and clinical doctors for cancer research. Findings indicate that down-regulates the expression of MMP-9 inhibits the growth and metastasis of tumor tissues [27]. MMP-9 involves in Naringin-mediated glioblastoma cells growth through the inhibition of ERK-P38-JNK signaling pathway [28]. Inhibition of MMP-9 gene expression in glioblastoma cell line through RNA interference leads to reduce tumor cells migration, invasion, growth and angiogenesis [29]. Interestingly, inhibitors p38 could arrest C6 glioblastoma cells and attenuate pro-inflammatory cytokine production and the invasiveness of human U251 [30,31]. Inhibition of JNK potentiates temozolomide-induced cytotoxicity in U87MG glioblastoma cells via suppression of AKT phosphorylation [32,33]. In this study, our results showed that Paclitaxel inhibits MMP-9, p38 and JNK expression in glioblastoma cells, suggesting MMP-9 is a potential target for the treatment of glioblastoma.
In conclusion, findings in the current study have found that Paclitaxel inhibits growth and proliferation of glioblastoma cells both in vitro and in vivo. We found that Paclitaxel induces apoptosis of glioblastoma cells via regulation apoptosis-related gene expression. Notably, observations indicate that paclitaxel suppresses growth and proliferation through down-regulation of MMP-9-mediated p38/JNK signaling pathway in glioblastoma cells, which contributes to understand molecular mechanism medicated by Paclitaxel in the progression of glioblastoma.
References
- Linsenmann T, Westermaier T, Vince GH, Monoranu CM, Lohr M. Primary spinal glioblastoma multiforme with secondary manifestation as a cerebral angioglioma. Literature review and case report. J Neurol Surg Rep 2015; 76: e128-134.
- Wang K, Kievit FM, Jeon M, Silber JR, Ellenbogen RG, Zhang M. Nanoparticle-mediated target delivery of trail as gene therapy for glioblastoma. Advanced Healthcare Materials 2015; 4: 2719-2726.
- Hariri OR, Quadri SA, Farr S, Gupta R, Bieber AJ. Third ventricular glioblastoma multiforme: case report and literature review. J Neurol Surg Rep 2015; 76: e227-232.
- Delia A, Fazzolari B, Arcovio E. Transdural spread of glioblastoma with endonasal growth in a long-term survivor patient: case report and literature review. Turkish Neurosurgery 2016; 26: 799-804.
- Capdevila C, Rodríguez Vázquez L, Martí J. Glioblastoma multiforme and adult neurogenesis in the ventricular-subventricular zone: a review. J Cell Physiol 2016.
- Delgado-Lopez PD, Corrales-Garcia EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Trans Oncol Off Publ Federation Spanish Oncol Soc Nat Cancer Institute Mexico 2016; 18: 1062-1071.
- Xhumari A, Rroji A, Enesi E, Bushati T, Sallabanda Diaz K. Glioblastoma after AVM radiosurgery. Case report and review of the literature. Acta Neurochir (Wien) 2015; 157: 889-895.
- Ong BY, Ranganath SH, Lee LY. Paclitaxel delivery from PLGA foams for controlled release in post-surgical chemotherapy against glioblastoma multiforme. Biomaterials 2009; 30: 3189-3196.
- Karmakar S, Banik NL, Ray SK. Combination of all-trans retinoic acid and paclitaxel-induced differentiation and apoptosis in human glioblastoma U87MG xenografts in nude mice. Cancer 2008; 112: 596-607.
- Nikanjam M, Gibbs AR, Hunt CA, Budinger TF, Forte TM. Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme. J Controlled Release 2007; 124: 163-171.
- Merighi S, Benini A, Mirandola P. Hypoxia inhibits paclitaxel-induced apoptosis through adenosine-mediated phosphorylation of bad in glioblastoma cells. Molecular Pharmacol 2007; 72: 162-172.
- Woo IS, Eun SY, Kim HJ. Farnesyl diphosphate synthase attenuates paclitaxel-induced apoptotic cell death in human glioblastoma U87MG cells. Neuroscience Letters 2010; 474: 115-120.
- Bonomi A, Lisini D, Navone SE, Frigerio S, Dossena M. Human CD14+ cells loaded with Paclitaxel inhibit in vitro cell proliferation of glioblastoma. Cytotherapy 2015; 17: 310-319.
- Bai FL, Yu YH, Tian H. Genetically engineered Newcastle disease virus expressing interleukin-2 and TNF-related apoptosis-inducing ligand for cancer therapy. Cancer Biology Ther 2014; 15: 1226-1238.
- Goudar RK, Shi Q, Hjelmeland MD. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther 2005; 4: 101-112.
- Delfino KR, Serao NV, Southey BR, Rodriguez-Zas SL. Therapy-, gender- and race-specific microRNA markers, target genes and networks related to glioblastoma recurrence and survival. Cancer Genom Proteom 2011; 8: 173-183.
- Han X, Chen J, Jiang M. Paclitaxel-paclitaxel prodrug nanoassembly as a versatile nanoplatform for combinational cancer therapy. ACS Appl Materials Interfaces 2016; 8: 33506-33513.
- Fukuchi M, Mochiki E, Ishiguro T, Ogura T, Sobajima J. Efficacy of nab-paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Anticancer Res 2016; 36: 6699-6703.
- Zhang T, Luo J, Fu Y. Novel oral administrated paclitaxel micelles with enhanced bioavailability and antitumor efficacy for resistant breast cancer. Colloids Surfaces B Biointerfaces 2016; 150: 89-97.
- Karpel-Massler G, Banu MA, Shu C, Halatsch ME, Westhoff MA. Inhibition of deubiquitinases primes glioblastoma cells to apoptosis in vitro and in vivo. Oncotarget 2016; 7: 12791-12805.
- Daniele S, Barresi E, Zappelli E. Long lasting MDM2/translocator protein modulator: a new strategy for irreversible apoptosis of human glioblastoma cells. Oncotarget 2016; 7: 7866-7884.
- Lalier L, Cartron PF, Pedelaborde F. Increase in PGE2 biosynthesis induces a Bax dependent apoptosis correlated to patients survival in glioblastoma multiforme. Oncogene 2007; 26: 4999-5009.
- Han MH, Kim SO, Kim GY. Induction of apoptosis by sanguinarine in C6 rat glioblastoma cells is associated with the modulation of the Bcl-2 family and activation of caspases through downregulation of extracellular signal-regulated kinase and Akt. Anti -Cancer Drugs 2007; 18: 913-921.
- Jiang Z, Zheng X, Rich KM. Down-regulation of Bcl-2 and Bcl-xL expression with bispecific antisense treatment in glioblastoma cell lines induce cell death. J Neurochem 2003; 84: 273-281.
- Bae IH, Lee WS, Yun DH, Han YH, Lee JS. 3-hydroxy-3, 4-dimethoxyflavone suppresses Bcl-w-induced invasive potentials and stemness in glioblastoma multiforme. Biochem Biophys Res Commun 2014; 450: 704-710.
- Lee WS, Woo EY, Kwon J. Bcl-w enhances mesenchymal changes and invasiveness of glioblastoma cells by inducing nuclear accumulation of beta-catenin. PloS one 2013; 8: e68030.
- Hu YH, Yu LJ, Shao ED, Wu JL, Ji JW. The regulating role of mutant IkappaBalpha in expression of TIMP-2 and MMP-9 in human glioblastoma multiform. Chinese Medical J 2009; 122: 205-211.
- Aroui S, Aouey B, Chtourou Y, Meunier AC, Fetoui H, Kenani A. Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP-2 and MMP-9) via the inhibition of ERK-P38-JNK signaling pathway in human glioblastoma. Chem Biol Interact 2016; 244: 195-203.
- Lakka SS, Gondi CS, Yanamandra N. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene 2004; 23: 4681-4689.
- Yeung YT, Bryce NS, Adams S. p38 MAPK inhibitors attenuate pro-inflammatory cytokine production and the invasiveness of human U251 glioblastoma cells. J Neuro-Oncol 2012; 109: 35-44.
- Yao YQ, Xu YH, Lu J, Zhou HY, Wang YZ. Effect of p38 MAPK on elemene-induced cell cycle arrest in C6 glioblastoma cells. Zhonghua Yi Xue Za Zhi 2008; 88: 56-58.
- Vo VA, Lee JW, Lee HJ, Chun W, Lim SY, Kim SS. Inhibition of JNK potentiates temozolomide-induced cytotoxicity in U87MG glioblastoma cells via suppression of Akt phosphorylation. Anticancer Res 2014; 34: 5509-5515.
- Liu QR, Liu JM, Chen Y. Piperlongumine inhibits migration of glioblastoma cells via activation of ROS-dependent p38 and JNK signaling pathways. Oxidative Med Cell Longevity 2014; 2014: 653732.