- Biomedical Research (2014) Volume 25, Issue 1
Oxidative Stress in Type II Diabetes Mellitus.
Mahendra D. Bikkad1*,Sachin D. Somwanshi2,Sandeep H. Ghuge3,N. S. Nagane41Associate Professor, Dept of Biochemistry, MIMSR Medical College, Latur, Maharashtra, India
2Associate Professor, Dept of Physiology, MIMSR Medical College, Latur, Maharashtra, India
3Assistant Professor, Dept. of Physiology, MIMSR Medical College, Latur, Maharashtra, India
4Associate Professor, Dept of Biochemistry, B.V. D. University Medical College and Hospital, Sangli Maharashtra, India
- *Corresponding Author:
- Mahendra D. Bikkad
Department of Biochemistry
MIMSR Medical College
Latur (Pin code- 413531) Maharashtra
India
Accepted November 18 2013
Citation: Bikkad MD, Somwanshi SD, Ghuge SH, Nagane NS. Oxidative Stress in Type II Diabetes Mellitus. Biomedical Research 2014; 25 (1): 84-87.
Abstract
Oxidative stress has been implicated in Diabetes mellitus. The oxidative stress is in terms of serum lipid peroxidase level and antioxidant activity in terms of superoxide dismutase and glutathione peroxidase levels were compared in type II Diabetes mellitus and age matched healthy controls. The mean values of serum lipid peroxidase were increased and superoxide dismutase and glutathione peroxidase levels were decreased in type II Diabetes mellitus as compared to controls. The oxidative stress was increased in type II Diabetes mellitus.
Keywords
Diabetes mellitus (DM), glutathione peroxidase, lipid peroxidase, Oxidative Stress, Superoxide dismutase
Introduction
Diabetes mellitus (DM) is a chronic disorder resulting from a number of factors in which an absolute or relative deficiency of insulin or its function occurs. The most common and life threatening disorder that besets type II diabetic subjects is coronary heart disease (CHD). Irrespective of the ethnic background, the risk for CHD among diabetic subjects is greater by a factor of 2 to 4 compared to non-diabetic subjects [1]. Coronary artery disease (CAD) is the third leading cause of mortality all over the world. Coronary Heart Disease (CHD) is a complex phenomenon with multiple risk factors that are either modifiable or non modifiable. Age, gender and genetic predisposition are non-modifiable risk factors. Diabetes mellitus is one of the major risk factor for CAD and other risk factors for CAD are hypertension, obesity, smoking and dyslipoproteinemias [2].
An oxidative stress in relation with the risk factors for CAD before the late advanced symptoms has been important point in recent research. Oxidative stress is defined as an imbalance between the production of reactive oxygen species (ROS) or free radicals and antioxidant defense, which may induce tissue injury. It can be assessed by measurement of reaction products of oxidative damage, like lipid peroxidation, DNA oxidation and protein oxidation [3]. During this study, serum MDA (malondialdehyde) levels are measured which is supposed to be an index of lipid peroxidation. In diabetes, oxidative stress is caused by both an increased formation of plasma free radicals and a reduction in antioxidant defenses. Hyperinsulinemia and hyperglycemia may enhance the production of free radicals and induce oxidative stress that may also contribute to increased risk for coronary artery disease in diabetes [4].
An unbalanced excess of free oxygen radicals due to lack of antioxidants may increase the risk of coronary artery disease. Superoxide dismutase is a naturally occurring enzyme that protects the body against active oxygen free radicals by scavenging excess superoxide. Lower undetectable levels of superoxide dismutase allow oxygen radicals to form in anaerobic bacteria and to inactivate other bacterial enzyme systems. SOD has particular value as an antioxidant that can help to protect against cell destruction [5]. The glutathione peroxidase enzyme takes part in a system that converts intra cellular free radicals in to less reactive or neutral components. Thus oxidative stress may play important role in pathogenesis of CAD risk factors. So the present study was planned to evaluate the levels of lipid peroxide & antioxidants in type 2 diabetes mellitus.
Material and Methods
Subjects
The present study was carried out in the Department of Biochemistry S.R.T.R. Medical College, Ambajogai. The cases were selected from those attended, the Medicine OPD, during the period January 2004 to December 2006 at S.R.T.R. Medical College, and Hospital, Ambajogai. The investigations were carried out in Biochemistry Laboratory, S.R.T.R. Medical College, and Hospital, Ambajogai.
Design
The total number of subjects included in the study was 80 and divided into two groups. Group I was consisting 40 normal healthy subjects as controls while group II was consisting 40 patients with Type II diabetes (Non-Insulin Dependent Diabetes Mellitus - NIDDM) as cases. The study included subjects of age in between 30-60 years for both groups.
Criteria for Selection
NIDDM (Type II Diabetes Mellitus) = Fasting Blood sugar level >140 mg/dl and postprandial blood sugar level > 200 mg/dl.
Methodology
Under all aseptic precautions 10 ml of morning blood sample was collected from anticubital vein of controls & cases after an overnight fasting. Five ml blood was collected in the bulb with heparin and 5ml were collected in plane bulb. Blood samples were centrifuged at 3000 rpm for 10 minutes. Heparinized whole blood was collected for estimation of enzyme RBC superoxide dismutase, RBC glutathione peroxidase while serum was used for estimation of lipid peroxide.
1. Estimation of Blood Sugar was done by GOD/ POD method. [6].
2. Serum Lipid Peroxide (MDA) was estimated by k. Satoh method [7].
3. Superoxide Dismutase activity was measured by using RANSOD method by randox laboratories ltd [8].
4. Glutathione peroxidase activity was measured by using RANSEL method by randox laboratories ltd [9].
Statistical Analysis
The biochemical parameters were expressed as mean + SD. Statistical significance was evaluated by student’s t - test. P value of less than 0.001 and 0.05 was considered to indicate statistical significance.
Results
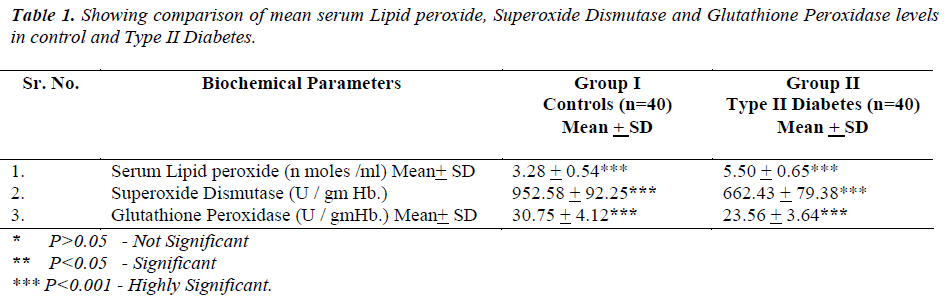
Our objectives were to evaluate the levels of serum lipid peroxide, erythrocyte superoxide dismutase (SOD) and erythrocyte glutathione peroxidase (GPx) in Type II Diabetes Mellitus. Type II Diabetes Mellitus cases (5.50+0.65 nmoles/ml) showed statistically significant (P < 0.001) increase in mean serum lipid peroxide level (MDA) as compared to controls (3.28+0.54 nmoles/ml). We also found statistically significant (P < 0.001) increase in mean erythrocyte SOD level in cases (662.43+ 79.38 U / gm Hb) as compared to controls (952.58+ 92.25 U / gm Hb) as well as statistically significant (P < 0.001) increase in mean erythrocyte GPx level in cases (23.56+ 3.64 U / gm Hb) as compared to controls (30.75+ 4.12 U / gm Hb) (Table 1)
Discussion
There has been considerable interest in the concept that the uncontrolled lipid peroxidation is a key contributing factor in the pathophysiology of CAD especially in Type II Diabetes. Lipid peroxidation eventually is a sequence of the injury caused by reactive oxygen species. Free
radical damages can accumulate over time and may thereby contribute to cell injury and development of human diseases. Free radicals have been implicated in the development of several diseases including atherosclerosis, diabetes, hypertension, obesity [10,11].
In present study we observed significant increase in the levels of lipid peroxide in Type II Diabetes as compared to the control group. The prolonged exposure to hyper glycemia also leads to the increased oxidative stress. Therefore study also made an attempt to estimate the levels of malondialdehyde, a marker of lipid peroxidation and found that levels of lipid peroxide were higher in diabetics compared to controls. Similar results were reported by Uzel et al (1987) [12], Gallow et al (1993) [13], Ayden (2001) [14] and Seghrolchni et al (2002) [15] in type II DM patients. Diabetes is a risk factor for atherosclerosis which is progressive and associated with enhanced oxidative stress. Hyperglycemia can induce oxidative stress by several different mechanisms. Autooxidation of glucose and the non enzymatic glycation of proteins generate superoxide (O2¯ ) [16]. Chatterjee SN stated that the permeability of the membrane has also been found to be increased in parallel with the lipid peroxidation indicating that the interior of the membrane undergoes alterations [17].Wolff SP et al stated that free radicals and oxidative stress may act as a common pathway to diabetes itself as well as to its later complications & found significantly higher lipid peroxide levels in diabetic than healthy individuals, matching our study results [18]. P. Ashok Kumar and G. Rajagopal observed that the level of TBARs in the erythrocytes was increased by 50% showing a significant generation of free radicals in the erythrocytes of diabetic patients [19]. Higher levels of lipid peroxide were observed in diabetic subject with vascular complication which may be due to the increased activity of the free radical formation. Free radical interacts in archidonic acid metabolism forming a toxic endoperoxidase. The lipid peroxide thus formed stimulates the synthesis of cyclooxygenase, prostaglandin and thromboxane which in turn causes increased platelets aggregation leading to vascular complications [20].
We found significantly lower erythrocyte SOD level in Type II Diabetes cases as compared to controls. SOD is considered to be the first line defense against the oxidative stress. Formation of free radicals in humans is considered to be related to several diseases, particularly CAD, but is controlled by various antioxidant protective mechanisms. These include primary enzymatic defenses such as superoxide dismutase and glutathione peroxidase [21,22]. Palanduz S, Ademoglu E et al observed that the SOD activity significantly decreased in diabetes mellitus. They suggested that there seems to be an imbalance between plasma oxidant and antioxidant systems in patients with type II diabetes [23]. Abdolijalal Marjani et al also found decreased erythrocyte SOD enzyme activity with type II diabetes as compared to controls [24].
We also found significantly decreased levels of erythrocyte GPx in Type II Diabetes cases as compared to controls. GPx activity was among the strongest predictors of the risk of cordiovascular events. Enzymatic inactivation of reactive oxygen species is achieved mainly by glutathione peroxidase, superoxide dismutase, and catalase. Glutathione and the glutathione peroxidase constitute the principal antioxidant defense system [25]. Memisogullari R et al reported that glutathione peroxidase activity was significantly decreased in type II diabetes mellitus. They suggested that the antioxidant deficiency and excessive peroxide mediated damage may appear in non insulin dependent diabetes mellitus [26]. Palanduz S, Ademoglu E et al reported that some complications of diabetes mellitus are associated with increased activity of free radicals and accumulation of lipid peroxidation products. The organism susceptibility to free radical stress and peroxidative damage depends upon the balance between the free radical load and the adequacy of antioxidant defenses. They found significantly decreased glutathione peroxidase activity in type II –diabetes [23]. Our results are comparable with the results reported by Palanduz S et al [23], Memisogullari et al [26], Martim and Colleagues [27].
Summary
An imbalance between oxidants and antioxidants is seen in diabetes mellitus which is the basic cause for CAD. Present study showed significantly elevated levels of lipid peroxide (MDA) in patients at risk for CAD. This increase in lipid peroxide may be due to the increased activity of the free radical formation. A deficiency of the antioxidant activity of superoxide dismutase and glutathione peroxidase has been related to higher concentration of lipid peroxide. There may be imbalance between production and scavenging free radical produced due to lack of antioxidant system. The estimation of lipid peroxide along with superoxide dismutase & glutathione peroxidase in diabetes mellitus is very useful as it may serve as a useful monitor to judge the prognosis of the patients. The detection of risk factors in the early stage of disease will help the patient to improve and reduce the morbidity rate. Thus we propose that along with clinical examination, estimation of oxidative stress may serve as supportive additional biochemical markers for early diagnosis and therapeutic intervention. Therapy of supplementation of antioxidants may prevent the risk of CAD in Type II Diabetes.
References
- Surekha Rani H, Madhavi G, Rao VR, B.K.Sahay and A. Jyothy. Risk factors for coronary heart dis- ease in type II dibetes mellitus. Indian Journal of clinical Biochemistry 2005; 20(2): 75-80.
- Judith R. MC Namara, Leo J. Seman, Ernst J. Schaefer. The laboratory’s role in identifying lipid and lipoprotein risk factors for CHD. Coronary heart disease, Medical Laboratory observer, Oct. 1999; 31(10);24-8,30-6,51;quiz38-9
- Halliwell B. Antioxidants and human disease: a general introduction, Nutr Rev 1997; 55: 44 – 49.
- Ceriell A. Oxidative stress and glycemic regulation.Metabolsim 2000; 49: 27-29.
- Math C, Glenz Y, Klaus M, Radermacher P, Speit G, Leverve X. Influence of an orally effective SOD on hyperbaric oxygen related cell damage. Free Radical Research 2004; 9: 927-932.
- Bayer Diagnostic India Ltd. Estimation of glucose by GOD / POD method. Autopak Kit. Trinder P. Ann. Clin Biochem 1969; 624
- Satoh K. Plasma lipid peroxide in cerebrovascular disorder determined by a new colorimetric method. Clinica Chimica Acta 1978; 90: 37-43.
- Woolliams JA, Wiener G, Anderson PH, McMurray CH Research in Veterinary Science 1983, 34; 253-256.
- Paglia DE, Valentine WN. Studies on the quantita-tive and qualitativecharacterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967; 70; 158-169.
- Delanty N, Dichetr MA. Oxidative injury in the nervous system. Acta Neurol Scand 1998; 98:145- 153.
- Sastre J, Pallardo FV, Corcia de la Asuncion J, Vina J. Mitochondria, oxidative stress and aging. Free Radic Res 2000; 32: 189-198.
- Uzel N, Sivas A, Uysal M and O, ZH. Erythrocyte lipid peroxidation and glutathione peroxidase activities in patients with diabetes mellitus, Horm Metab Res.1987; 19, 89-90.
- Gallou G, Ruelland, A Legras, B Maugendre D, Allannic, H and cloarec L. Plasma malondial- dehyde in type I and type II diabetic patients. Clin Chem. Acta.1993; 214, 227-234.
- Aydin A, Ortan H, Sayal A, Ozata M, Sahin G, and Isimer A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: effects of glycemic control. Clin Bio Chem.2001; 34, 65-70.
- Seghrouchni I, Dral J, and Bannier E. Oxidative stress parameters in type I and type II and insulin treated type II diabetes mellitus: Insulin treatment efficiency Clin Chem. Acta.2002; 321, 89-96.
- Baynes JW. Role of oxidative stress in develop- ment of complications in diabetes, Diabetes. 1991; 40: 405-412.
- Chatterjee SN, Agrawal S, Amitkumar. Membrane lipid peroxidation and its pathological conseque- nce. Ind J Biochem and Biophysics.1988; 25: 25 – 31.
- Wolff SP, British Medical Bulletin.Diabetes mellitus and free radicals.1993; 49(3): 642-652.
- P. Ashok Kumar and G. Rajagopal. Lipid peroxida- tion in erythrocytes of patients with Type 2 diabe-tes mellitus. Indian journal of clinical biochemis- try. 2003; 18(1): 71-74.
- N.P. Suryawanshi, A.K. Bhutey, A.N. Nagdeote,A.A. Jadhav and G.S. Manoorkar. Study of lipid peroxide and lipid profile in diabetes mellitus. Indian journal of clinical Biochemstry. 2006; 21(1): 126-130.
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine 3rd ed. Oxford: Oxford University Press; 1999.
- Mc Call MR, Frei B. Can antioxidant vitaminsmaternally reduce oxidative damage in humans? Free Radic Biol Med 1999; 26: 1034-1053.
- Palanduz S, Ademoglu E, Gokkusu C, Tamer S. Res Commun Mol pathol pharmocol 2001; 109 (56): 309-318.
- AbdolJalal Marjani. Plasma lipid peroxidation zinc and erythrocyte Cu – Zn superoxide dismutase enzyme activity in patients with type 2 diabetes mellitus in Gorgan city (south east of the Caspian Sea). The Internet Journal of Endocrinology. 2005; 2(1). DOI: 10.5580/126b.
- Stefan Blankenberg, Hans J, Ruppercht, ChristophBickel, Michael Torrewski, Gerd Hafner, Laurence Tiret, Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. NLJM.2003; 349: 1605-1613.
- Memisogullari R, Taysi S, Bakan E, Capogly I. Antioxidant status and lipid peroxidation in type II diabetes mellitus. Cell Biochem 2003; 21(3): 291-296.
- Martim AC, Sanders RA, and Watkins JB, 3rd: Diabetes, oxidative stress, and antioxidants: Areview. J Biochem Mol Toxicol. 2003 17(1): 24-38.