- Biomedical Research (2011) Volume 22, Issue 2
Oxidative Stress and Antioxidant Status in Patients of Ovarian Cancer
Sanjyoti Bandebuche1*, R. R. Melinkeri2
1Department of Biochemistry, Maharashtra Institute of Medical Education and Research (MIMER Medical College), Talegaon Dabhade, Pune, Maharashtra, India.
2Department of Biochemistry, Bharati Vidyapeeth Deemed University Medical College, Pune, Maharashtra, India.
- *Corresponding Author:
- Sanjyoti Bandebuche
Department of Biochemistry
Maharashtra Institute of Medical Education and Research (MIMER Medical College), Talegaon Dabhade
Pune, Maharashtra
India.
Accepted date: December 31 2010
Abstract
Ovarian cancer has the highest mortality rate amongst all gynecologic malignancies. A major role in its initiation and progression is played by DNA damage, mostly caused by oxidative stress. The presented study was designed to determine whether oxidative stress plays an important role in ovarian malignancy. Serum malondialdehyde (MDA) was measured as an indicator of lipid peroxidation and antioxidant status was assessed by estimating serum vitamin E and erythrocyte superoxide dismutase (SOD) levels. Finally, the status of antioxidants and lipid peroxidation was correlated with the pathophysiology of ovarian cancer. The study comprised of thirty age-matched controls and sixty ovarian cancer patients - thirty patients of stage II and thirty of stage IV, all selected by transvaginal sonography and confirmed by histopathological examination. The results reveal that serum MDA levels were increased, while serum vitamin E and erythrocyte SOD levels decreased, as the disease progressed from stage II to IV. These results suggest that the increased serum MDA levels indicate oxidative stress which may cause DNA damage which is one of the causative factors for cancer. Low levels of vitamin E and SOD could be due to the increased utilization of these antioxidants in scavenging the lipid peroxides, production of which overrides the antioxidant defense leading to increased MDA in serum.
Keywords
Ovarian cancer, oxidative stress, antioxidants, malondialdehyde (MDA), vitamin E, superoxide dismutase.
Introduction
In India, 15% of all gynecological cancers are ovarian malignancies [1]. It has the highest mortality rate amongst all gynecologic malignancies [2,3]. It is a disease of peri-menopausal and post-menopausal women; however no age is exempt from ovarian neoplasm. There is no socio-economic bar for ovarian malignancy but epidemiologically it is more frequent in higher socio-economic and industrially developed countries [4].
Risk factors for ovarian carcinoma include inflammation, excessive number of life time ovulations, increase in steroid hormone levels, infertility, age, asbestos, talc and reproductive factors such as nulli-parity [5].
At the time of diagnosis, over 85% of patients with ovarian cancer present with advanced stage III or IV disease characterized by intraperitoneal, lymphatic, and/or distant spread of disease; the poor prognosis associated with ovarian cancer is attributed to a lack of symptoms at early stages of the disease as well as a lack of biomarkers for the detection of early stage disease. Moreover, despite appropriate surgery and receiving highly effective first-line chemotherapy approximately 20-30% of patients with advanced stage disease continue to have evidence of residual disease during treatment and never have a complete clinical response [6]. Early diagnosis of ovarian malignancy is most important for better prognosis. A high index of suspicion, better screening modalities and recognition of high risk factors help to detect ovarian malignancy earlier [4].
Ovarian Cancer and Oxidative Stress
Oxidative stress is due to a disturbance in the balance between the production of reactive oxygen species (ROS) and efficiency of the antioxidant defense. In other words, oxidative stress results if excessive production of ROS overwhelms the antioxidant defense system or if there is a significant decrease or lack of antioxidant defense [7]. Potential biological targets for free radical attack include lipids, proteins and nucleic acids [8].
The majority of cancers of the ovary are thought to originate from a surface epithelial cell perturbed by ovulation [9]. Reactive oxidants (hydroxyl radicals, superoxide radicals, hydrogen peroxide, and singlet oxygen) generated during the mechanics of ovulatory follicular rupture, damage the DNA of ovarian surface epithelial cells that are located within limited diffusion radius [10].
Recent molecular studies have shown that ovarian cancer has acquired genetic alterations of oncogenes and tumor suppressor genes such as BRCA1, p53, nm23 and K-ras, which may be due to inflammation and oxidative stress [11].
Mutagenic capacity of free radicals is due to the direct interaction of hydroxyl radicals (OH-) with DNA as it reacts indiscriminately with all components of the DNA molecule. Whereas, superoxide radicals (O2) and hydro-gen peroxide do not directly react with DNA, rather it is the hydroxyl radicals produced after their interaction with transition metals, which are responsible for DNA damage [12]. By contrast, singlet oxygen (1O2) selectively attacks guanine. The most commonly produced base lesion and the most often measured as an index of oxidative DNA damage is 8-hydroxy guanine (8-OHG). Conversion of guanine to 8-hydroxy guanine, a frequent result of reactive oxygen species attack has been found to alter the enzyme-catalyzed methylation of adjacent cytosines, thus providing a link between oxidative DNA damage and altered methylation patterns as methylation of cytosines in DNA is important for the regulation of gene expression [13].
Oxidative stress induces a cellular redox imbalance which has been found to be present in various cancer cells compared with normal cells, the redox imbalance thus may be related to oncogenic stimulation. DNA mutation is a critical step in carcinogenesis and elevated levels of oxidative DNA lesions (8-OH-G) have been noted in various tumors, strongly implicating such damage in the etiology of cancer. It appears that the DNA damage is predominantly linked with the initiation process [14].
Moreover, severe oxidative stress is not only known to cause DNA damage and mutations of tumor suppressor genes which are initial events in carcinogenesis [7], but can also play an important role in the promotion of multi-step carcinogenesis [15,16]. Hence the study was planned to see the effect of oxidative stress in causation and progression of ovarian cancer.
Aims and Objectives
The main objective of the study was to see the effect of oxidative stress in causation and progression of ovarian cancer patients. So the study was planned under following aims:
1. To measure serum malondialdehyde (MDA) levels as an indicator of oxidative stress (lipid peroxidation) in ovarian cancer patients.
2. To measure serum vitamin E and erythrocyte superoxide dismutase levels as an indicator antioxidant status.
3. To correlate the status of antioxidants and lipid peroxidation in the pathophysiology of ovarian cancer.
Materials and Methods
The present study comprised of sixty newly transvaginal ultrasonographically diagnosed ovarian cancer patients confirmed by histopathological examination at B J Medical College and Sasoon General Hospital, Pune, Maharashtra, India, ranging in the age group of 50-70 years. Ovarian carcinomas were staged according to the classification of International Federation of Gynaecology and Obstetrics (FIGO) and as the occurrence of patients in stage II and stage IV were common in this hospital, they were included in the study. The controls and subjects were categorized into:
Group A: Thirty healthy individuals with normal transvaginal USG findings and pelvic examination served as controls.
Group B: Thirty patients with stage II carcinoma ovary (tumour involving one/both ovaries with pelvic extension)
Group C: Thirty patients with stage IV carcinoma ovary (growth involving one/both ovaries with distant metastases in liver, lungs and pleura)
All the patients and controls were free from any other gynaecologic malignancies like breast, cervical, uterine cancer and also any concomitant illness such as diabetes mellitus, cardiovascular disease and liver disease etc. Informed consent was obtained from the ovarian cancer patients and the normal subjects before study. The present study was also approved by the human ethical committee, B J Medical College and Sasoon General Hospital, Pune, Maharashtra, India.
Sample Collection
About 5 ml of fasting venous blood was collected with all aseptic precautions in plain bulb. Separated serum was used for measurement of MDA (an index of lipid peroxidation) and vitamin E (an antioxidant vitamin).
About 2 ml of blood was collected in ethylene diamine tetracetic acid (EDTA) bulb. Plasma separated by centrifuging at 2500 revolution per minute for 10 minutes and the erythrocytes washed thrice with normal saline. Afterwards the erythrocytes were lysed with equal volume of distilled water. This haemolysate was used for measurement of superoxide dismutase (an antioxidant enzyme).
Biochemical Estimations
Lipid peroxidation was assessed by the measurement of MDA in serum by the method of Buege et al using thiobarbituric acid reaction [17]. Vitamin E was estimated by the method of Baker and Frank [18]. Erythrocyte superoxide dismutase was estimated by Marklund and Marklund method [19].
The biochemical data are expressed as mean ± standard deviation. Statistical significance was analyzed using unpaired ‘t’ test.
Results
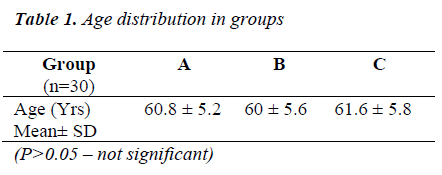
Table 1 shows that all the groups were statistically comparable (P>0.05) for the age distribution.
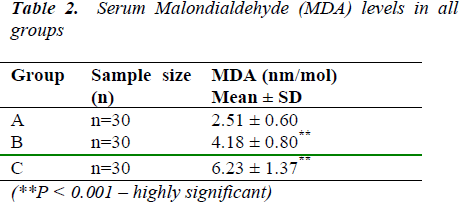
Table 2 shows the rise in MDA levels was found to be highly significant in both groups of ovarian cancer patients than in control individuals (2.51 ± 0.60 nmol/ml). The rise was also highly significant in stage IV ovarian cancer patients (6.23± 1.37 nmol/ml) than stage II patients (4.18±0.80 nmol/ml).
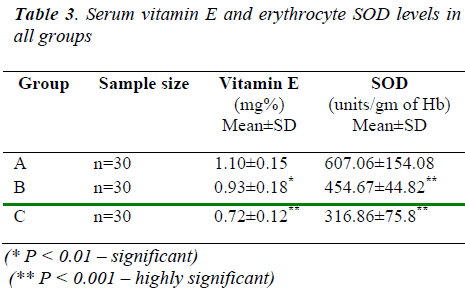
Table 3 shows the fall in vitamin E levels was found to be significant in both groups of ovarian cancer patients than control (1.10 ± 0.15 mg%). The fall was also highly significant in stage IV ovarian cancer patients (0.72 ±0.12 mg%) than stage II patients (0.93 ±0.18 mg%). And it also shows that the fall in SOD levels was found to be highly significant in both groups of ovarian cancer patients than in control subjects (607.06 ± 154.08 units/gm of Hb). The fall was also highly significant in stage IV ovarian cancer patients (316.86 ±75.8 units / gm of Hb) than stage II patients (454.67 ± 44.82 units/ gm of Hb).
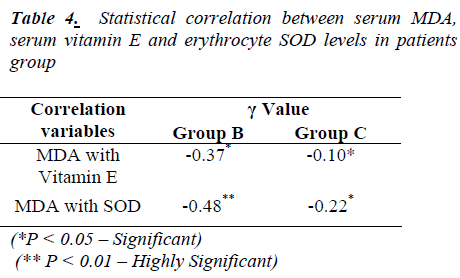
Table 4 shows significant negative correlation between MDA and vitamin E in group ‘B’ while it is highly significant between MDA and SOD. In group ‘C’, there is significant negative correlation between MDA and Vitamin E, also between MDA and SOD levels
Results of this study were comparable with [20,21].
Discussion
Lipids, especially polyunsaturated fatty acids (PUFA) are very susceptible to free radical attack which can initiate lipid peroxidation [22]. Lipid peroxidation plays an important role in the control of cell division [23]. The end product of lipid peroxidation, malondialdehyde (MDA) due to its high cytotoxic and inhibitory action on protective enzymes is suggested to act as a tumor promoter and a co-carcinogenic agent [24]. Since MDA is an index of lipid peroxidation, it was estimated in patients with ovarian cancer to estimate the extent of lipid peroxidation. In present study, increased levels of MDA in the circulation of ovarian cancer patients can be attributed to increase in oxidative stress due to the deficiency of antioxidant mechanism.
The antioxidant enzyme superoxide dismutase (SOD) widely distributed in all cells is present in high amounts in erythrocytes [25]. Superoxide dismutase, the antioxidant enzyme plays an important role in scavenging the superoxide radical. SOD protects cells againstsuperoxide radical by dismutation of the highly reactive superoxide anion to oxygen and to a less reactive oxygen species, hydrogen peroxide [26].
In present study, the observed increase in circulating lipid peroxides of ovarian cancer patients correlate with the decline in SOD activity. This can result in accumulation of superoxide anion, highly diffusible and potent oxidizing radical capable of traversing membranes causing deleterious effects at site far from the tumor.
Vitamin E (also called as alpha- tocopherol) is the most effective (by acting as a hydrogen donor at its six hydroxyl 6-OH group), chain breaking antioxidant in cellular membranes and thereby contributes to membrane phospholipids stability and safeguards intracellular molecules against damage produced by free radicals [27,28,29]. It is essentially non-toxic. In present study, the decreased levels of serum vitamin E may be due to their increased utilization in scavenging lipid peroxides as well as sequestration by tumor cells [30]. Epidemiology studies support an inverse relationship between circulating levels of vitamin E with ovarian cancer [31,32]. Due to low economic status, women were malnouri-shed and thus more prone to malignancy. Studies indicate that women who consume fewer amounts of fruits and vegetables are associated with ovarian cancer [33]. Thus the enhanced lipid peroxidation observed in ovarian cancer patients can also be attributed to a large extent to the depletion of vitamin E in the diet.
From above discussion, it becomes clear that, thus low levels of SOD and vitamin E in the ovarian cancer patients may be due to increased utilization to scavenge lipid peroxides as well as their sequestration by tumor cells. Increased levels of lipid peroxidation may be due to excessive oxidative stress caused by incessant ovulation or epithelial inflammation initiators such as talc and asbestos which is increased as the disease progresses from stage II to stage IV while levels of antioxidants decreased.
The imbalance between free radicals and antioxidants results in oxidative stress. This plays an important role in causation and progression of ovarian cancer by causing structural alterations in DNA directly or indirectly through the formation of genotoxic lipid peroxidation byproducts that reacts with DNA. This probably leads to lipid peroxidation related carcinogenicity.
Antioxidants (SOD and vitamin E) appear to ease oxidative stress by scavenging reactive oxygen species before they cause damage to the various biological molecules or prevent oxidative damage from spreading e.g. by interrupting the radical chain reaction of lipid peroxidation.
Our findings provide evidence for antioxidants such as vitamin E significantly contributes to antioxidant defense thus vitamin E together with a diet high in vegetables and fruits might reduce the risk of ovarian cancer until more evidence become available.
Inadequate data is available on the antioxidants and lipid peroxidation status in patients with ovarian cancer. Hence additional studies are needed to substantiate these suggestions.
References
- Chhabra S, Sonak M, Prem V, et al. Gynaecological Malignancies in a Rural Institute in India. J Obstet Gynaecol 2002; 22(4):426-9.
- O’Rourke J, Mahon SM, et al. A Comprehensive Look at the Early Detection of Ovarian Cancer. Clin. J. Oncol Nurs. 2003; Vol.7 (1):41-7.
- Edwards BK. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 116:544-73.
- Howkins and Bourne. Diseases of Ovary. Shaws Textbook of Gynaecology 1994, 11th edition, 442.
- Roberta B. Ness and Carrie Cottreau. Possible Role of Ovarian Epithelial Inflammation in Ovarian Cancer. J National Cancer Institute, 1999; 91:17, 1459-1467.
- Nossov V, The early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125? Am J Obstet Gynecol 2008; 199 :215-23.
- Kang DH. Oxidative Stress, DNA Damage, and Breast Cancer. AACN Clin Issues 2002; 13:540-9.
- Pryor WA. Cancer and Free Radicals. Basic Life Sci. 1986; 39: 45-59.
- William J Murdoch and James F Martinchick. Oxidative Damage to DNA of Ovarian Surface Epithelial Cells Affected by Ovulation: Carcinogenic Implication and Chemoprevention. Experimental Biol-ogy and Medicine, 2004; 229; 546-552
- Cancer Risk Factors. Ovulation Stress might Cause Ovarian Cancer. Cancer Weekly 2005.
- Horiuchi A, Itoh K, Shimizu M, et al. Toward Unders-tanding the Natural History of Ovarian Carcinoma Development: A Clinicopathological Approach. Gyn-aeol Oncol.2003; 88(3): 309-17.
- Free Radical Mechanisms in Cancer Formation. The Doctors Lounge-Articles, 2002.
- Helen Wiseman and Barry Halliwell. Damage to DNA by Reactive Oxygen and Nitrogen Species: Role in Inflammatory Disease and Progression to Cancer. Biochem. J. 1996, 313, 17-29.
- Valko M, Rhodes CJ, Monocol J, et al. Free radicals, Metals and Antioxidants in Oxidative Stress-induced Cancer. Chem Biol Interact, 2006; 160: 1-40
- Ahmed MI, Fayed ST, Hossein H, et al. Lipid Peroxidation and Antioxidant Status in Human Cervical Carcinoma. Dis Markers. 1999; 15(4): 283-91.
- Ahmed Abdal Dayem, Hye-Yeon Choi, Jung-Hyun Kim, et al. Review Role of Oxidative Stress in Stem, Cancer, and Cancer Stem Cells. Cancers 2010;2(2):859-884
- Buege and Aust. Microsomal Lipid Peroxida-tion.Methods in Enzymology, 1978; 105:302-310
- Baker H and Frank O. Determination of Serum Alpha-Tocopherol, 1968. Gowenlock AH, McMurray JR, Mchauchian DM: Varley’s Practical Clinical Biochemistry; 6th Edition, London; 902-903.
- Marklund SL and Marklund G. Involvement of Superoxide Anion Radical in the Auto-oxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. European J Biochem, 1974; 47:469-474.
- Kumarasamy S, Selvaraj A, Namasivayam N, et al. Evidence of Oxidative Stress in the circulation of Ovarian Cancer Patients. Clinica Chimica Acta, 2004; 339:27-32.
- Anbazhagan M, Chellappan PR, Activities of Antioxidant Enzyme and Lipid Peroxidation in Ovarian Cancer Patients, Academic Journal of Cancer Research 2009;2(2):68-72
- Halliwell B, Gutteridge MC. Free Fadicals in Biology and Medicine. Third Edition London: Oxford : 1999.
- Diplock AT, Rice-Evans AC, Burton RH. et al. Is There a Significant Role for Lipid Peroxidation in the Causation of Malignancy and for Antioxidants in Cancer Prevention? Cancer Res 1994; 54: 19525-65.
- Otamiri T and Sjodahl R. Increased Lipid Peroxidation in Malignant Tissues of Patients with Colorectal Cancer. Cancer 1989; 61: 122-5.
- Speranza MJ, Bagley AC, Lynch RE. et al. Cells Enriched for Catalase are Sensitized to the Toxicities of Bleomycin, Adriamycin, and Paraquat. J Biol Chem. 1993; 268 (25): 19039-43.
- McCord JM. The Evolution of Free Radicals and Oxidative Stress. Am J Med 2000; 108: 652-9.
- National Research Council. Vitamin Tolerance of Animals. Washington, DC: National Academies Press, 1987.
- Morrissey PA and Sheelay PJA. Optimal Nutrition: Vitamin E. Proc Nutr Soc, 1999; 58:459-468.
- Herrera E and Barbas C. Vitamin E: Action, Metabolism and Perspectives.J Physiol Biochem 2001; 57:43-56.
- Manju V, Kalaivani SJ, Nalini N. et al. Circulating Lipid Peroxidation and Antioxidants Status in Cervical Cancer Patients: A Case Control Study.Clin Biochem. 2002; 35(8): 621-5.
- Bidoli E, La Vecchia C, Talamini R, et al. Micronutrients and Ovarian Cancer: A Case Control Study in Italy.Ann Oncol. 2001; 12(11):1589-93.
- Aaron TF, Sara HO, Laura M, et al. Dietary Antioxidant Supplements, and Risk of Epithelial Ovarian Cancer. Nutr Cancer 2002; 40:92-8.
- Eastwood MA. Interaction of Dietary Antioxidants in Vivo: How Fruits and Vegetables Prevent Disease? QIM 1999; 92: 527-30.