- Biomedical Research (2011) Volume 22, Issue 3
Oxidative stress adversely affects spermatogenesis in male infertility
Z.G. Badade, Kavita More and Jayashree NarshettyDepartment of Biochemistry,MGM Medical College, Kamothe,Navi-Mumbai, India
Department of Gynecology and Obstetrics, MGM Medical College, Kamothe,Navi-Mumbai, India
- *Corresponding Author:
- Musa ACAR
Mevlana University
Department of Physical Therapy and Rehabilitation
42003 Konya TURKEY
Accepted February 14 2011
Abstract
Oxidative stress is one of the factors which affects fertility potential of spermatozoa by lipid peroxidation which may lead to sperm dysfunction. Sperm count and sperm motil-ity are prime parameters that determine the functional ability of spermatozoa. The present study was aimed to study adverse effect of oxidant on spermatogenesis in idiopathic male in-fertility. The study includes total 60 subjects, Normospermic (n=30) and Oligoastheno-spermic (n=30). Seminal plasma malondialdehyde, nitric oxide, zinc and SOD were esti-mated by spectrophotometric methods and correlated with sperm count and sperm motility. Seminal levels of malondialdehyde and nitric oxide were significantly higher (p<0.001) while zinc and SOD were significantly lower (p<0.001) in oligoasthenospermic than normospermic men. There was significant negative correlation between malondialdehyde and nitric oxide with sperm count and motility in oligoasthenospermia. The increase in seminal malondial-dehyde, nitric oxide and decrease in zinc and superoxide dismutase levels in oligoastheno-spermia may be responsible for disruption in the membrane integrity of spermatozoa and have role in reduction of sperm DNA integrity and DNA damage. The positive correlation of zinc with sperm count and sperm motility indicates an important role of zinc in spermato-genesis. Thus these parameters could be useful for determining sperm fertilization potential further in diagnosis, prognosis and treatment of male infertility especially in idiopathic male infertility
Keywords
Oxidants, antioxidants, zinc, oligoasthenospermia.
Introduction
Human male infertility is the major health problem worldwide [1]. In last ten years there has been tremendous scientific growth in the field of reproductive medicine. Its prevalence in Western countries has been estimated with 20%. As per WHO study, incidence of infertility in India is 10 to 15%. In 15% infertile couples, about 30% cases are due to males only and in another 20% both partners have detectable abnormalities. Thus a male factor plays a significant role in about 50% of infertile couples [2-3].
Infertility is defined as failure to conceive after one year of regular unprotected intercourse with the same partner. Infertility has been attributed to number of factors such as anatomic defects, endocrinopathies, immunologic prob-lems, gene mutation, radiation exposure, chemotherapy, ejaculatory failures and environmental exposures. Causes cannot be found in over 25% of infertile males, and these cases are referred to as idiopathic infertility [4]. In many such cases oxidative stress has been implicated as a major causative factor.
Oxidative stress (OS), results from accumulation of ex-cessive reactive oxygen species (ROS), during spermato-genesis epididymal sperm maturation as well as from ex-posure of toxic chemicals, environmental pollutants etc. ROS change lipid/protein ratio of membranes by affecting polyunsaturated fatty acids and lipid peroxidation (LPO) causes functional irregularities of several cellular organ-elles [5,6]. It is therefore essential to understand role of such free radical mediated sperm damage mechanisms that could induce male infertility. Scavenging of these free radicals during spermatogenesis by means of antioxi-dants could provide a promising approach to suppress such damage.
Generally male infertility is assessed by semen analysis which provides a basic data regarding reproductive male function [7]. Sperm count and sperm motility are first and most important predictors of fertility [8]. Seminal plasma malondialdehyde is the stable peroxidation product; its estimation is a simple to evaluate the effect of peroxida-tion on sperm [9]. The nitrogen derived free radicals, ni-tric oxide (NO·) and peroxynitrite anion (ONOO·) also play significant role in the reproduction and fertilization.
High amounts of NO· have detrimental effects on sperm [10]. Zinc also plays an important role in normal testicu-lar development, spermatogenesis and sperm motility [7]. With a view of understanding the potential role of oxida-tive stress in idiopathic male infertility, the present study was undertaken. We aimed to assess seminal plasma lev-els of malondialdehyde, nitric oxide, zinc and SOD activ-ity in idiopathic oligoasthenospermic patients and nor-mospermic group and to find out their correlation with sperm count and sperm motility.
Materials and Methods
Present study was carried out in the Department of Bio-chemistry, Department of Obstetrics and Gynecology, MGM Medical College, Kamothe, Navi Mumbai. Thirty primary infertile male subjects aged 21-45 years, without any treatment, who had regular unprotected intercourse for at least 12 months without conception with their part-ner, were selected. The institutional ethical committee clearance was obtained for the present study. Cases with leukocytospermia, Varicocele, hypogonadism, history of smoking, alcohol and prolonged illness were excluded from the study. Wives of the infertile subjects included had no obvious causes of infertility like tubal blockage or ovulation disorders. At first clinic attendance, a detailed background history and physical examination were done on both husband and wife.
The 60 semen samples were divided into two groups: Group I - Normospermic (n=30), Group II – idiopathic Oligoasthenospermic (n=30). The subjects having sperm count less than 20 million/ml and sperm motility less than 50% were considered as oligoasthenospermic, while thirty fertile males aged 21-45 years, whose partners had con-ceived within a year and having sperm count more than 20 million/ml with motility more than 50% in forward progression were selected from general population and considered as normospermic control group.
Semen samples were analyzed according to WHO criteria. Samples were collected by masturbation in wide mouth sterile plastic container after minimum of three days of abstinence. On due orientation about the nature of study, a written consent was obtained from healthy individuals & infertile male subjects. After liquefaction, samples were processed by conventional analysis to determine sperm count, sperm motility according to WHO criteria.
On centrifugation, seminal plasma was used for meas-urements of malondialdehyde by Satoh K method [11]. Nitric oxide was estimated by Griess reaction. In this ki-netic method, nitrate is reduced to nitrite by copper coated cadmium granules. This nitrite produced was determined by diazotization of sulfanilamide and coupling to napthyl-enediamine [12]. Zinc was estimated by spectrophotomet-ric method (kit by Coral Clinical systems); zinc in alka-line medium reacts with nitro-para amino phospho sul-phate to form purple colored complex. The color intensity is directly proportional to the amount of zinc present in the sample. SOD was measured according to the method of Marklund and Marklund [13]. Superoxide anion radical is involved in the auto-oxidation of pyrogallol. At alkaline pH, SOD dismutates superoxide, thereby inhibiting the auto oxidation of pyrogallol.
Statistical analysis
Statistical analysis of the data was carried out with SPSS, version 16; Data was reported as mean ± SD. The com-parisons between two groups were tested by unpaired t-test. A 95% confidence interval was used. P values less than 0.05 were considered statistically significant. Corre-lation between two continuous outcomes was evaluated using Pearson correlation coefficients.
Results
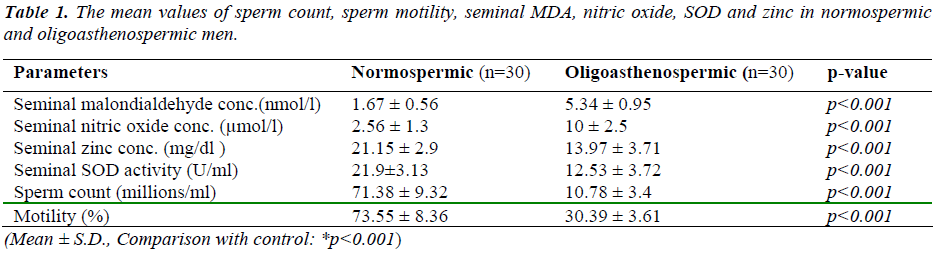
Results were expressed as mean ± SD for each parameter. Statistically significant differences among oligoastheno-spermic and normospermic groups are indicated in Table No.1 along with their significant values. Seminal levels of malondialdehyde (5.34 ± 0.95 nmol/l) and nitric oxide (10 ± 2.5 μmol/l) were significantly high (p<0.001) in oli-goasthenospermic patients than normospermic men (1.67 ± 0.56, 2.56 ± 1.3 respectively). Seminal levels of zinc (13.97± 3.71mg/dl) and SOD (12.53 ± 3.72 U/ml) were significantly lower (p<0.001) in oligoasthenospermic men than normospermic men (21.15 ± 2.9, 21.9±3.13 respec-tively).
Discussion
Impairment of spermatogenesis and sperm function is the most common causes of male infertility. Abnormal sperm function is difficult to evaluate and treat. There is a lack of understanding of the factors contributing to normal and abnormal sperm function leading to infertility [14]. Pre-sent routine investigations are insufficient to predict exact cause of infertility. Recent studies showed oxygen-derived free radicals, induce damage to spermatozoa. Several studies reported that Idiopathic male factor infer-tility has been linked with oxidative stress [15,16] Pasqualotoo et al reported men with idiopathic infertility have higher levels of ROS and lower antioxidant proper-ties than healthy controls [17]. The main cause for this connection is the observation of morphologically abnor-mal sperm, commonly identified in the idiopathic infertile male population and a reduced antioxidant capacity [18].
Mammalian spermatozoa membrane is rich in polyunsatu-rated fatty acids. This makes them very susceptible to oxygen-induced damage, which is mediated by lipid per-oxidation. In a normal situation, the antioxidant mecha-nisms present in the reproductive tissues are likely to quench these reactive oxygen species (ROS) and protect gonadal cells and mature spermatozoa from oxidative damage [14].
Unlike somatic cells, mature spermatozoa lack cytoplasm. Since cytoplasm is the major source of anti-oxidants, lack of cytoplasm in the mature spermatozoa causes deficiency in both oxidant defense and endogenous repair mecha-nism, however naturally the deficiency of antioxidant sys-tem is compensated by the enzymatic and non-enzymatic antioxidants in seminal plasma [19]. So decreased anti-oxidant enzyme or increased ROS level disrupts the physiological functions of the spermatozoa and impairs sperm motility and process of fertilization [20].
The purpose of our study was to evaluate the adverse ef-fect of oxidant on spermatogenesis in idiopathic male in-fertility and to find out their correlation with sperm count and sperm motility.
Previous studies reported that lipid peroxidation plays significant role in impairing sperm functions and semen quality especially sperm count, motility and morphology. Excess ROS and low antioxidant levels in semen en-hanced susceptibility of sperm DNA to denaturation and might cause mitochondrial DNA mutations which impairs the fertilizing capacity of spermatozoa [21,22].
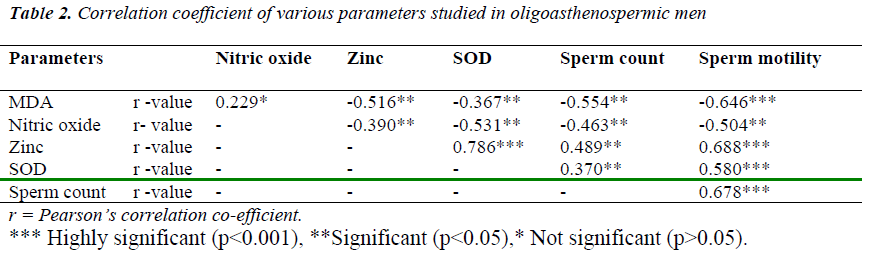
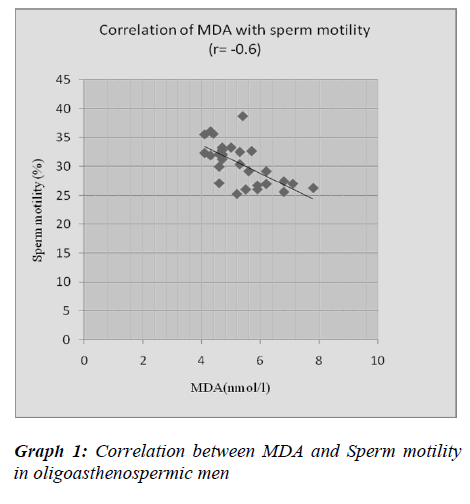
In present study, we found high levels of MDA in oli-goasthenospermic patients as compared to normospermic and it is negatively correlated with sperm count and sperm motility. Our results of MDA are on par with stud-ies by Hsieh YY et al. [23] and Fraczek et al. [24]. Frac- zek et al. [24] reported elevated seminal MDA concentra-tion in patients with oligoasthenoteratozoospermia. Our results are in contrast with Suleiman et al. [25]. They demonstrated that MDA concentration in the seminal plasma was not related with the sperm concentration and motility.
Amiri et al. [10], Sheikh et al. [26] reported significantly higher values of seminal nitric oxide in infertile males as compared to fertile males. Giancarlo et al. [27] demon-strated same findings in idiopathic asthenospermia. Fur-ther they observed significant negative correlation be-tween NO. concentration and sperm motility. In contrast, Revelli et al. [28] observed that NO. concentration in the seminal plasma was not correlated with sperm concentra-tion and motility. We observed the negative correlation of the NO. concentration with sperm count and motility.
Nitric oxide is a biological messenger. It is produced by one of the essential amino acids L-arginine, by the cata-lytic action of enzyme NO synthase (NOS). It’s overpro-duction in seminal plasma may be due to induced genital tract cells such as Leydig cells, epididymal or vas defer-ence, epithelial cell or spermatozoa itself and also ob-served in sub infectious or inflammatory diseases of male genital tract and by induced leucocytes [10]. In the pre-sent study, source of NO. is unknown and it may be pro-duced by macrophages in response to infection, or from steady secretion from multiple sources such as testis and structures of male reproductive tract [29].
The high concentration of NO. may react with superoxide ion and hydrogen peroxide resulting in the formation of peroxynitrite, hydroxyl radical which cause oxidation of sperm membrane lipids and thiol-proteins [30]. It can cause reduction of sperm DNA integrity, sperm DNA damage and fragmentation of sperm DNA, which are main factors in male infertility. NO. may also inhibit cel-lular respiration by nitrosylation of heme in mitochondrial enzymes, aconitase and glyceraldehyde phosphate dehy-drogenase leading to depletion of adenosine triphosphate (ATP) and consequent loss of motility by spermatozoa [27].
Oligoasthenospermia is associated with high levels of MDA and nitric oxide. It suggests that lipid peroxidation of the membrane lipid may disturb the functions carried out by the sperm membrane. There was significantly negative correlation of MDA and Nitric oxide with sperm motility suggesting their role in inhibition of mitochon-drial function and reduction of membrane fluidity which is necessary for sperm-oocyte fusion.
Omu et al. [31] had demonstrated that zinc therapy results in significant improvement in sperm quality with increase in sperm density, progressive motility and improve con-ception and pregnancy outcomes. It appears to be a potent scavenger of excessive superoxide anions produced by defective spermatozoa and/or leukocytes in human semen after ejaculation [32]. Insufficient intake of zinc can im-pair antioxidant defenses and may be an important risk factor in oxidant release, compromising the mechanism of DNA repair and making the sperm cell highly susceptible to oxidative damage. In the absence of Zn, the possibility of increased oxidative damage exists that would contrib-ute to poor sperm quality. Decrease of seminal Zn and poor Zn nutrition can be a risk factor for sperm abnormal-ity and idiopathic male infertility [33].
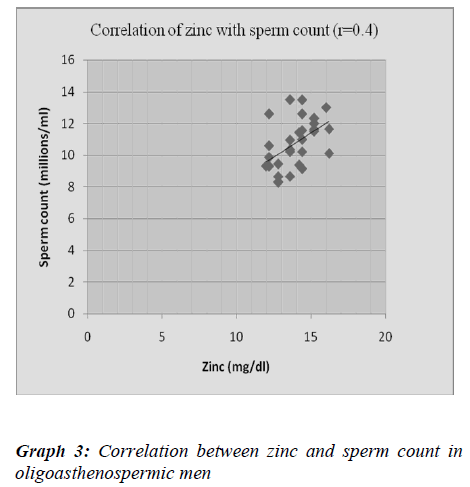
Chia et al. [34] reported significant positive correlation of zinc concentration with sperm density, motility and vi-ability. Fuse H et al. [35] demonstrated lower levels of zinc in oligoasthenospermic male which was a positively correlated with sperm concentration and sperm motility. Wong WY et al. [36] reported no correlation of zinc con-centration with sperm quality or with male infertility. In present study, we observed significantly low levels of zinc in oligoasthenospermic patients and showed signifi-cantly positive correlation with sperm count and sperm motility. Zinc act as an essential element for spermato-genesis, motility and scavenger of superoxide anion. The low zinc levels may contribute to low sperm concentra-tion and poor motility.
SOD is an important antioxidant of seminal plasma which has superoxide scavenging capacity and plays an essential role in maintaining the balance between ROS generation and degradation [37]. Several toxic substances including chemical reagents, drugs, heavy metal ions or nicotine decrease semen quality as well as SOD activity in seminal plasma [38].
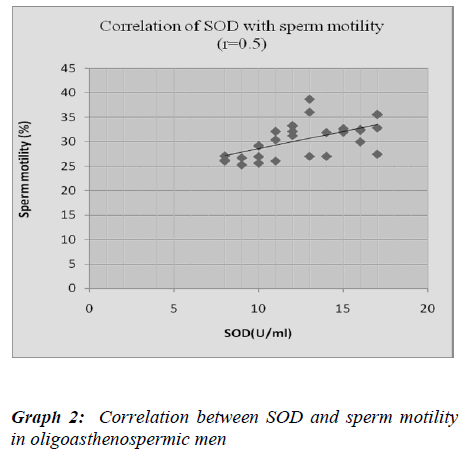
We found significantly lower seminal SOD activity in oligoasthenospermic compared to normospermic. Our results are in accordance with Murawski et al. [38], Sicili-ano et al. [39]. In present study, SOD activity showed significantly positive correlation with sperm count and sperm motility which was compatible with Murawski et al [38]. Murawski et al. [38] got same findings in oligoas-thenospermia. Storey BT et al. [40] demonstrated com-plete loss of motility in the sperm sample and it was di-rectly proportional to the SOD activity. This strongly suggests SOD activity is insufficient to cope with the ex-cessive amount of ROS and Reactive nitrogen species.
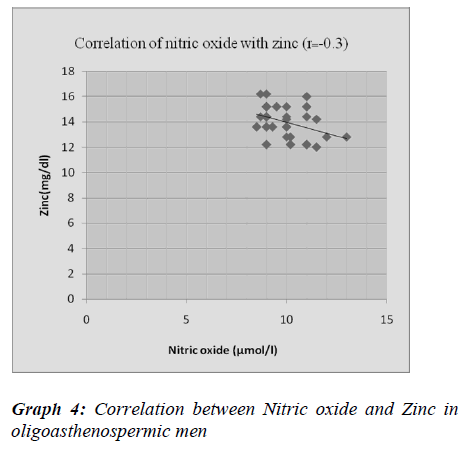
There was negative correlation between malondialdehyde and nitric oxide with zinc and SOD in oligoasthenosper-mia, indicating oxidative stress can cause male infertility by affecting the process of conception at various stages, like damaging the sperm membrane, DNA and protein. It may impair normal sperm function as well as sperm ca-pacitation and acrosome reaction, which are essential for fertilization.
Thus our study suggests evaluation of oxidative stress and antioxidant status can be considered as additional parame-ters of male infertility especially in idiopathic cases. The threshold ROS levels above which antioxidants can be used for the treatment of male infertility can be deter-mined, which will be helpful in the management of infer-tility.
Conclusion
The increase in malondialdehyde, nitric oxide and de-crease in zinc and superoxide dismutase levels in oligoas-thenospermic men may cause disruption in the membrane integrity of spermatozoa and may have role in reduction of sperm DNA integrity. There was a positive correlation of zinc with sperm count and sperm motility, indicating zinc plays an important role in spermatogenesis. Thus estimation of seminal malondialdehyde, nitric oxide, zinc and SOD can be useful tool for determining sperm fertili-zation potential. These parameters could assist in diagno-sis and treatment of male infertility especially in idio-pathic cases.
Acknowledgement
We are extremely thankful to MGM University of Health Sciences, Kamothe, Navi Mumbai- 410209 for the financial support for present work.
References
- World Health Organisation. Towards more objectivity in diagnosis and management of male infertility, Int J Andrology 1987; 7: 1-35.
- Singh MP, Sinha MBK, Sinha R. Male infertility : Medicine Update 2005; 318-322
- Gopalkrishnan K. Decreasing sperm counts-fact or fic- tion. ICMR Bulletin, 1997, 27: (8).
- Campbell AJ, Irvine DS. Male infertility and intracyto-plasmic sperm injection (ICSI). Br Med Bull 2000; 56: 616-629.
- Agarwal A and Said MT. Oxidative stress DNA dam- age and apoptosis in male infertility: a clinical ap- proach.BJU International; 2005, 95: 503-507
- Tremellen K Oxidative stress and male infertility—aclinical perspective Human Reproduction Update. 2008; 14: 243-258
- Limthong P, Thanida P. Zinc levels in seminal plasma of infertile Man. Srinagar Ind Med J, 2005; 20 (1): 38-42
- Venkatesh S, Singh G, Gupta NP, Kumar R, Deecara- man M, Dada R. Correlation of sperm morphology and oxidative stress in infertile men Iranian Journal of Re- productive Medicine, 2009; 7 (1): 29-34,
- Geva E, Lessing JB, Lerner-Geva L, Amit A. Free radicals, antioxidants and human Spermatozoa: Clinical implications. Hum. Reproduction, 1998; 13:1422-1424
- Amiri I., Sheike N., Najafi R. Nitric oxide level in seminal plasma of fertile and infertile males and its cor- relation with sperm parameters. DARU, 2006; 14 (4) 197-202
- Sathoh K. Serum lipid peroxide in cerebrovascular dis- orders determined by a new colorimetric method, Clin Chim Acta, 1978; 90: 37-43.
- Najwa KC and Nabil WW. Determination of inorganic nitrate in serum and urine by kinetic cadmium reduc- tion method. Clinical Chemistry, 1990; 36(8):1440-1443.
- Marklund S, Marklund G, Involvement of the superox- ide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase, Eur J Sikka SC, Relative impact of oxidative stress on malereproductive function. Current Medicinal Chemistry, 2001; 8: 851-862
- Said TM, Agarwal A, Sharma RK, Thomas AJ, Jr, Sikka SC. Impact of sperm morphology on DNA dam- age caused by oxidative stress induced by beta- nicoti- namide adenine dinucleotide phosphate. Fertil Steril 2005; 83:95–103.
- Joffe M,What has happened to human fertility? Human Reproduction, 2010 25 : 295-307
- F. Pasqualotto , R. Sharma H Kobayashi, D Nelson, A Jr. Thomas and A. Agarwal.Oxidative stress in nor-mospermic men undergoing infertility evaluation, J Androl 2001; 22: 316-322.
- Garrido N, Meseguer M, Simon C, Pellicer A, RemohiJ. Prooxidative and anti- oxidative imbalance in human semen and its relation with male fertility. Asian J An- drol 2004; 6: 59-65.
- Agrwal A & Allamaneni S S, Oxidants and antioxi- dants in human fertility. Middle East Fertil Soc J, 2004; 9: 187-197
- Rivlin J, Mendel J, Rubinstein S, Etkovitz N, BreitbartH. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol Reprod, 2004; 70:518-522
- Kumar R, Venkatesh S, Kumar M, Tanwar M, Shasmsi MB, Gupta NP, Sharma RK, Talwar P, Rima Dada,Oxidative stress and sperm mitochondrial DNA muta-tion in idiopathic oligoasthenozoospermic men, Indian Journal of Biochemistry and Biophysics, 2009; 46:172-177
- Nabil H, Lekaa A. Moemen and Manal H. Abu Elela.Studying the levels of malondialdehyde and antioxidant parameters in normal and abnormal human seminal plasma, Australian Journal of Basic and Applied Sci-
ences, 2008; 2(3): 773-778. - Hsieh YY, Chang CC, Lin CS, Seminal malondialde- hyde concentration but not glutathione peroxidase ac- tivity is negatively correlated with seminal concentra- tion and motility. Int J Biol Sci, 2006; 2:23-29
- Fraczek M, Szkutnik D, Sanocka D, Kurpisz M. Per- oxidation components of sperm lipid membranes in male infertility. Ginekol Pol. 2001; 72:73-9
- Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl, 1996; 17:530-7
- Nasrin Sheikh, Iraj Amiri. The correlation between total antioxidant capacity and Nitric Oxide concentra- tion in seminal plasma with sperm DNA damage. Afr J of Biotech, 2010; 9(35):5739-5745,
- Balercia G, Moretti S, Vignini A, Magagnini M, Mantero F, Boscaro M, Ricciardo-Lamonica G,Mazzanti L. Role of Nitric Oxide Concentrations on human Sperm Motility. Journal of Andrology, 2004; 25: 245–249
- Revelli A, Bergandi L., Massobrio M., Lindblom B, Bosila A, Ghiyo D. The concentration of nitrite in seminal plasma does not correlate with sperm concen- tration, sperm motility, leucocytospermia, or sperm cul- ture. Fertil. Steril; 2001; 76 (3): 469-500.
- Huang I , Jones J , Khorram O . Human seminal plasma nitric oxide: correlation with Sperm morphology and testosterone. Med Sci Monit. 2006 12(3): 103-106.
- Stamler JS, Singel DJ, Loscalzo J. Biochemistry ofnitric oxide and its redox activated forms. Science, 1992; 258: 1898 –1902
- Omu AE, Dashti H, Al-Othman S. Treatment of as- thenozoospermia with zinc sulphate: andrological, immunological and obstetric outcome.Eur J Obstet Gy-necol Reprod Biol, 1998; 79:179-184
- Gavella M, Lipovac V. In vitro effect of zinc on oxida- tive changes in human semen. Andrologia, 1998; 30:317–323.
- Colagar AH, Marzony ET, Chaichi MJ. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutrition Research, 2009; 29:(2), 82-88
- Chia SE, Ong CN, Chua LH, Ho LM, Tay SK. Com- parison of zinc concentration in blood and seminal plasma and various sperm parameters between fertileand infertile men. J Androl, 2000; 21: 53-57.
- Fuse H, Kazama T, Ohta S, Fujiuchi Y. Relationship between zinc concentration in seminal plasma and various sperm parameters. Int Urol Nephrol, 1999; 31:401-408.
- Wong WY, Flik G, Groenen PM, Swinkels DW, Thomas CM, Copius-Peereboom JH, Merkus HM, Steegers-Theunissen RP. The impact of calcium, mag- nesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod Toxicol, 2001; 15:131-6.
- de Lamirande E, Gagnon C. Human sperm hyperactiva- tion in whole semen and its association with low super- oxide scavenging capacity in seminal plasma. FertilSteril, 1993; 59(6):1291-1295.
- Evaluation of superoxide dismutase activity and its impact on semen quality parameters of infertile men. Folia Histochem Cy- tobiol, 2007; 45(1): 123-126
- Siciliano L, Tarantino P, Longobardi F, Rago V, De Stefano C, Carpino A. Impaired seminal antioxidant capacity in human semen with hyperviscosity or oli- goasthenozoospermia. J Androl, 2001; 22(5):798-803
- Storey BT. Biochemistry of the induction and preven- tion of lipoperoxidative damage in human spermatozoa. Mol HumReprod, 1997; 3(3): 203-213