Research Article - Journal of Gastroenterology and Digestive Diseases (2016) Volume 1, Issue 2
Overview of mucin (MUC13) in gastrointestinal cancers.
- *Corresponding Author:
- Brij K Gupta, DVM, Ph.D.
Banfield Pet Hospital Denton, USA
Tel: +6053709763
E-mail: brij_gupta77@yahoo.com
Accepted date: November 09, 2016
Abstract
Mucins are the most abundant high molecular weight O-glycosylated glycoproteins present in mucus. MUC13, a member of mucin protein family, is a membrane bound mucin. MUC13 has shown to be implicated in variety of gastrointestinal cancers including cancers of salivary glands (mucoepidermoid carcinoma), esophagus (esophageal squamous cell carcinoma), stomach (gastric adenocarcinoma), liver (intrahepatic hepatocellular carcinoma), pancreatic (pancreatic ductal cell carcinoma), and colorectal cancers (adenocarcinoma). In this review, we highlight the roles and implications of mucin MUC13 in various GI carcinogenesis. We also discuss the utility of MUC13 as diagnostic/prognostic indicators alone or in combination of other mucins/marker in GI cancers.
Keywords
Mucin, MUC13, Gastrointestinal cancers, Diagnostic/Prognostic indicator.
Abbreviations
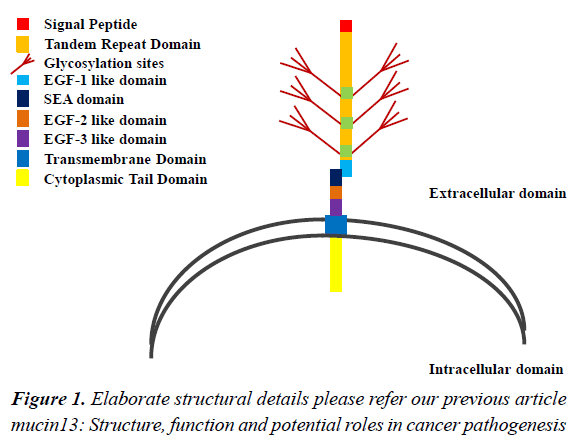
GIT: Gastrointestinal Tract; MEC: Mucoepidermoid Carcinoma; ESCC: Esophageal Squamous Cell Carcinoma; IHC: Intrahepatic Hepatocellular Carcinoma; IBD: Inflammatory Bowel Disease; IGD: Inflammatory Gastric Diseases; SP: Signal Peptide; EGF- 1: Epidermal Growth Factor 1; EGF-2: Epidermal Growth Factor 2; EGF-3: Epidermal Growth Factor 3; SEA domain: Sea urchin sperm protein, Enterokinase and Agrin; TM: Transmembrane Domain; CT: Cytoplasmic Domain.
Introduction
Mucins
Mucins are high molecular weight glycoproteins (0.5 MDa to 20 MDa) composed of approximately 80% carbohydrate and about 20% amino acid contents [1]. Mucins have been discovered decades ago and extensively studied not only for its role in cellular physiological properties such as lubrication and protection but also for various disease processes such as inflammatory and neoplastic conditions [2-4]. Mucins are being secreted through goblet cells of epithelial surfaces. They tend to adhere with epithelial surface, lubricate and form protective layer against dreadful environment pathogens. Mucins are normally expressed at low levels in the normal epithelial surfaces of gastrointestinal, respiratory, and reproductive tract organs [5-9] and protect these epithelial surfaces from obnoxious external insults by creating a physical barrier that preserves internal physiological environment and are essential for cell survival [10-13]. Besides their role in protective function for the normal epithelial surface of tissues, alterations in expression pattern of mucin are a common feature of several neoplasia [14,15]. The aberrant expression of mucin has been shown in multiple cancers [5-7]. One of the important features of mucin is the presence of a tandem repeat domains in which O- and N-glycosylation structure builds.Tandem repeats of various mucins are extensively studied [16]. Alterations in the expression and/or glycosylation patterns of mucins influence cellular growth, differentiation, transformation, cell adhesion, cell invasion and immune surveillance [17,18]. Cytoplasmic tail contains serine, threonine and protein kinase C phosphorylation motif site which are potential phosphorylation site to initiate cell signaling. Multiple studies have established a clear connection between mucin and multiple GI malignancies such as pancreatic, esophageal, salivary gland, gastric and hepatobiliary and colorectal cancers etc. [19-24]. In addition, mucins have been also well studied as diagnostics and prognostic indicator in GI cancers.
Classification of Mucins
Until now, 20 mucin family members have been studied and designated as MUC1, MUC2, MUC3A-B, MUC4, MUC5AC, MUC6, MUC7, MUC8, MUC9, MUC11, MUC12, MUC13, MUC15, MUC16, MUC17 and MUC19, MUC20, MUC21. Structurally, mucins share extracellular (protrudes exterior to the cell surface), transmembrane (embedded within cell membrane) and cytoplasmic tail domain (located at the interior of cell surface). Based on structurally and functionally resemblances mucins are broadly classified into three broad groups. First group is composed of MUC2, MUC5B, MUC5AC, and MUC6. These mucins are predominantly secretory or gel forming mucins. The second group of mucins is composed of MUC1, MUC3A, MUC3B, MUC4, MUC11, MUC12, MUC13, MUC15, MUC16, MUC17, MUC20 and MUC21. These mucins are loosely attached with cell surface and secreted in the mucus. They are also referred as transmembrane or membrane bound mucins because they harbor a transmembrane domain and a short cytoplasmic tail domain. The third subgroup composed of MUC7, MUC8, and MUC9. These mucins are exclusively secreted non-gel forming mucins (soluble mucins) (Table 1).
| Mucins family of proteins classification | ||
|---|---|---|
| Secretory/Gel forming | Membrane bound/Transmembrane | Soluble |
| MUC2 | MUC3A | MUC7 |
| MUC5AC | MUC12 | MUC8 |
| MUC5B | MUC13 | MUC9 |
| MUC6 | MUC17 | |
| MUC19 | MUC20 | |
| MUC21 | ||
MUCIN13 (MUC13)
Table 1: Classification of Mucins family of proteins.
Mucin proteins were discovered several decades ago. However, MUC13 is a relatively newly discovered mucin. For the first time, William et al. identified a novel transmembrane mucin MUC13 by in silico search using serine, threonine rich search string in the GenBank EST data base [25]. This search identified a gene that was expressed in rodent bone marrow. The complimentary DNA (cDNA) sequence of the human orthologue of this gene was further identified and was found to be located at chromosome position 3q21.2 (GenBank accession number AF286113) and was flanked by genes ITGB5 (β integrin) and HEG-1 (heart of glass). Later, HUGO (human genome organization) gene nomenclature committee (HGNC) designated this gene as “MUC13”. The MUC13 mRNA contains 12 exons (2.8 kb length) and encodes 512 amino acids [26,27]. Structurally, from N terminus to C terminus, MUC13 consists of SP, TR (hallmark of mucins), EGF-1 like, SEA, EGF-2-3 like, and TM located at extracellular space and CT tail domain in the intracellular space (Figure 1) (for elaborate structural details please refer our previous article mucin13: structure, function and potential roles in cancer pathogenesis). MUC13 is a membrane bound mucin and has been studied in normal tissues as well as in various diseases including malignancy and inflammatory diseases [28-35]. The normal expression of MUC13 has been reported in epithelial surfaces of multiple organ system including esophagus, stomach, colon, trachea, kidney and some of the hematopoietic cells [26]. However, the over expression of MUC13 has been shown in cancers of esophageal, gastric, pancreatic, ovarian and colorectal origin [5,6,36,37]. In this review, we, in particular discuss the overview/current trends and clinical importance of mucins MUC13 in GI cancer carcinogenesis. We also discuss MUC13’s potential utility as diagnostic/prognostic marker and its correlation with patient survival.
Salivary Gland Cancer
Mucoepidermoid carcinoma (MEC) is the most common subtype of malignant salivary gland tumor. This kind of tumors are both epithelial and mucin type. To date, only one report is available pertaining to role of MUC13 in MEC. In this report, Semirani et al. showed expression profile and patient survival of MUC13 and other mucins members of mucin family including MUC1, MUC4, MUC12, MUC13, MUC17, MUC18 and MUC19 in 23 MEC patients and its adjacent normal counterpart using RT-PCR and quantitative PCR. Accordingly, majority (65%) of MEC tissues expressed MUC19. MUC1 and MUC4 were expressed 4.2 and 21 fold high in MEC. On the other hand, MUC13 was over-expressed in 13% of tumors and MUC12, MUC17 expression was not found. Based on these results authors concluded that no definite association for MUC13, MUC17-19 expression and patient survival was noted in their study. They also mentioned that because of small sample size no specific association was seen and therefore further study in a larger cohort of tissues are needed to draw definite conclusion. Despite, they also drew conclusion that MUC4 expression was predictive of more favorable prognosis whereas MUC1 and MUC19 expression was predictive of less favorable prognosis [38].
Esophageal Squamous Cell Carcinoma (ESCC)
ESCC is one of the common malignancy in US with estimated new cases of 16910 and estimated death 15,690 [39]. Other mucins have been extensively studied in ESCC, however, only two studies have been reported thus far in relation to MUC13’s role in ESCC carcinogenesis and its utility as biomarkers [40-42] . In the first study, Wang et al. investigated expression pattern of MUC13 in combination with MUC20 for its prognostic significance and patient survival in ESCC patients who received nonadjuvant chemotherapy after surgery. They performed IHC analysis in 186 patients and found that MUC13 and MUC20 expression was mostly localized in the cytoplasm. About 53.8% of patients had high MUC13 expression and about 38.2% patient had high MUC20 expression. However, neither MUC13 nor MUC20 expression was associated with long term patient survival when evaluated independently. In contrast, the median patient survival was significantly high in tissues expressing low MUC13 and high MUC20 expression when combined together. They concluded that MUC13 and MUC20 expressions together were an independent prognostic indicator in ESCC for the patients who had received neoadjuvant chemotherapy after surgery [43]. In another report, Shen et al. studied the gene expression profiling of ESCC patients to evaluate response to neoadjuvant chemotherapy using cDNA array technology and chemo sensitive and resistant cell lines. They found that almost 200 genes were differentially expressed, however, 6 genes including MUC13, MUC4 and MUC20 was selected in the study because of their role in tumor development and progression. Their results revealed that MUC4 and MUC20 were expressed at low levels and MUC13 were over expressed in chemo sensitive ESCC cell lines. Silencing MUC4 and MUC20 further increased the sensitivity of ESCC cell lines to neo-adjuvant chemotherapeutic agent whereas silencing MUC13 decreased the drug chemo sensitivity. All together, they concluded that blockage of MUC13 decreased chemo sensitivity and MUC4 and MUC20 could be utilized as potential biomarkers for predicting efficacy of neoadjuvant chemotherapy in ESCC patients [44].
Gastric Cancer
Gastric cancer is one of the devastating diseases with estimated new cases of 26,370 and estimated deaths are 10,730 in 2016 in US. Several mucins have been shown to play an important role in gastric carcinogenesis and have been shown to be determinant of prognosis [45-48]. Shimamura et al. for the first time, reported increased expression of MUC13 both at mRNA and protein levels in approximately 65% of gastric cancer cases. They also reported that MUC13 expression was predominant in the intestinal type gastric cancer whereas in intestinal metaplasia (precancerous lesions of intestinal type of gastric cancer) and in the normal gastric mucosa MUC13 expression was almost absent. Tubular glands of stomach showed apical membrane MUC13 staining whereas diffuse type gland showed more pronounced cytoplasmic staining. They also concluded that MUC13 expression was not correlated with clinico-pathologic factors such as depth of invasion and lymph node metastasis [37]. In another study, Lee et al. reported that there was a 115-fold increase in MUC13 expression in intestinal metaplasia compared to normal tissues. They also reported that cytoplasmic MUC13 expression may be associated with decreased survival rates among gastric cancer patients [49]. The same group of researcher conducted another study in which MUC13 expression was evaluated along with other metaplasia biomarkers and found that combined loss of expression of MUC13 along with other marker considered an independent prognostic indicator in undifferentiated gastric cancers [50]. In another study, Liu et al. showed that MUC13 expression disrupts gastric homeostasis resulting in the development of gastric metaplasia [51]. Altogether these studies suggest that MUC13 plays an essential role in gastric neoplasia and is an important prognostic indicator.
Hepatic Cancer
Intrahepatic duct cancer is the one of the top five causes of deaths in US with estimated new cases 39,220 and estimated deaths 27,170. Subrungruanga et al. studied the gene expression profile using microarray in Thai patients having intrahepatic cholangiocarcinoma associated with the infection with liver fluke parasite. Their result revealed that a 2,821 upregulated genes whereas 1,361 down regulated genes. Of those up regulated genes MUC13 was among them and was further confirmed using RTPCR. This report suggested that MUC13 along with other genes that were upregulated in this study can be used as novel genetic marker for classifying and for the early diagnosis of cholangiocarcinoma [52].
Pancreatic Cancer
Pancreatic cancer is a leading cause of death. While few reports are available pertaining to molecular mechanism of MUC13 in other gastrointestinal cancers, it is relatively well studied in pancreatic cancer [5]. Our group have previously shown over expression of MUC13 in pancreatic tumorigenesis [5]. In this study, immunohistochemical analysis in tissues of pancreatic intraepithelial (PanIN) lesions revealed significantly higher MUC13 expression in PanIN lesions whereas normal tissue showed undetectable MUC13 expression. High expression of MUC13 was correlated with well and moderately differentiated tumors. Exogenous expression of MUC13 in xenograft mouse model was associated with increased tumor volume whereas MUC13 knockdown reduced tumor volume. MUC13 expression also correlated with increased motility, invasion, and reduced adhesion of pancreatic cancer cells. In a recently published paper Khan et al have shown over expression of HER2 and its colocalization with MUC13 in indicating potential therapeutic target utility in subset of HER 2 expressing pancreatic ductal adenocarcinoma patients [53]. In a recently published report Khan et al. reported regulatory pathway of MUC13 via miRNA-145. They reported that MUC13 expression inversely correlates with miRNA-145 expression in pancreatic cancer cells and tumor tissues. They stated that MUC13 miRNA-145 targets 3’ untranslated region of MU13 and down regulates MUC13 expression. Their study suggests that miR-145 is a novel regulator of MUC13 expression in pancreatic cancer that will further help understand regulatory molecular mechanism which may potentially offer new avenue for treatment of pancreatic cancer [54].
Colorectal Cancer
Colorectal cancer is one of the most common forms of malignancy in the developed world. It is also the third and second leading cause of cancer related deaths in the USA and world, respectively [55]. It is estimated that, approximately 95,270 new cases will be diagnosed, of which, 49,190 estimated deaths due to colon cancer in 2016 [55]. In the first published report regarding expression of MUC13 in colon cancer, Walsh et al. determined the expression of MUC13 on various stages of colon cancer using immunohistochemistry (IHC) analysis to determine localization and expression of MUC13 in colorectal cancer tissues [36]. They reported that in the normal colon, MUC13 was detected on the apical membranous surface of glands and goblet cells with variable cytoplasmic MUC13 immunostaining. The highest MUC13 immuno-reactivity was observed on crypts and surface epithelium. MUC13 was highly expressed in 81% of well differentiated colon adenocarcinomas and 50% mucinous adenocarcinomas. MUC13 staining was more pronounced on the left side adenocarcinoma compared to right side. The poorly differentiated cancers had more cytoplasmic staining. There was also a trend towards poor survival rate in the patients whose tumors showing baso-lateral MUC13 expression. However, in contrast to these results, later, Packer et al. reported decreased MUC13 mRNA expression in colon cancer tissues [35]. The limitation of their study was that they conducted this study only at mRNA level and not at protein level therefore actual expression of MUC13 protein in colon cancer tissues were not determined [35]. In another report, a study using a digital gene expression displayer in a small number of patient samples revealed the presence of MUC13 mRNA in the blood of patients of colorectal cancer but not in the blood of normal healthy patients suggesting that MUC13 may be utilized as serum marker for colon cancer detection [56]. Our group had earlier shown that aberrant localization (cytoplasmic and nuclear) and increased MUC13 expression has also been correlated to poor tumor grade and metastasis in colon cancer cells indicating its utility as potential biomarker for colon cancer metastasis [7]. Additionally, our group had shown that over expression of MUC13 enhances tumorigenic properties of colon cancer cell [57]. For instance, functional assays in exogenously expressing MUC13 revealed that MUC13 drives cell proliferation, differentiation and augments tumorigenic properties in colon cancer [5,6,57]. We further showed that STAT5 binds with MUC13 upon binding of chemokine factor IL6 and regulates its expression in colon cancer cells. Altogether, our previous report suggested that MUC13 over-expression drives colon carcinogenesis via JAK2-STAT5 signaling pathway.
Our group earlier reported that over-expression of MUC13 enhanced ability of colon cancer cells to proliferate, migrate, invade and form colonies. In addition to colon carcinogenesis, it is also shown that MUC13 is also implicated in certain inflammatory and precancerous disease conditions. MUC13’s role has been studied in some of the GI inflammatory diseases such as IBD and IGD [58-60]. Sheng et al. have shown that when MUC13 knockout mice were subjected to a colitis inducing agent dextran sulfate sodium (DSS), the expression of MUC13 protected against intestinal epithelial damage and inflammation caused by DSS [61]. In another study, Sheng et al. reported that MUC13 silencing in LS513 intestinal epithelial cells decreased chemokine secretion in response to tumor necrosis factor-α. The pro-inflammatory activity of MUC13 was also seen after exposure to pathogens. MUC13 also regulated chemokine secretion in gastrointestinal epithelial cells through a nuclear factor-κB-dependent pathway. Sheng et al. further concluded that MUC13 is an important component of gastrointestinal homeostasis and the disruption or inappropriate expression of this mucin could predispose to infectious and inflammatory diseases and inflammation-induced cancers [60]. These reports suggest that, in addition to being involved in the development of multiple GI cancers, MUC13 plays an important role in certain GI inflammatory diseases as well [34].
Gastrointestinal Neuroendocrine Cancers
Small bowel (SBNTs) and pancreatic neuroendocrine tumors (PNETs) are relatively rare occurring tumors and incidence is gradually increasing. These tumors also have over 50% of metastasis rate. Carr et al. for the first time studied these tumors to evaluate gene expression profile for six target genes including G-protein-coupled receptor 113 (GPR113); oxytocin receptor (OXTR); secretin receptor (SCTR); adenosine-A1 receptor (ADORA1); meprin-A-beta receptor (MEP1B) and MUC13. In this study, mRNA profiling in 45 patients with primary and metastatic lesions compared to normal tissues revealed significant over-expression of oxytocin receptor (OXTR) in both primary and metastatic SBNETs and PNETs. MUC13 and MEP1B was overexpressed in PNET primary tumors, GPR113 was overexpressed in primary SBNETs and their metastases. SCTR and ADORA1 were significantly under-expressed in PNETs and their metastases. Altogether, their finding revealed that even though MUC13 and other genes are significantly overexpressed only OXTR may be a potential target for the diagnostic and therapeutic intervention for SBNETs and PNETs [62].
Conclusion
MUC13 is relatively newly identified mucin, despite, available reports points towards its implications/roles in various GI malignancies/inflammatory processes and its utility as a diagnostic/prognostic indicator either alone or in combination with other membrane bound proteins. However, except couple of reports [53,54] in pancreatic cancer and a single report in colon cancer [57], little is known about molecular/regulatory mechanism of MUC13 in other GI cancers carcinogenesis. Further research is required to decipher the complexity of molecular/regulatory mechanism of MUC13 in the pathogenesis of various GI malignancies and inflammatory diseases. This will also aid on bringing its potential utility as diagnostic/prognostic biomarker for early detection and development for targeted immunotherapy to minimize mortality associated with lethal GI cancers.
References
- Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77-99.
- Dong Y, Walsh MD, Cummings MC, et al. Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours. J Pathol. 1997;183:311-7.
- Roy LD, Sahraei M, Subramani DB, et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene. 2011;30:1449-59.
- Furr AE, Ranganathan S, Finn OJ. Aberrant expression of MUC1 mucin in pediatric inflammatory bowel disease. Pediatr Dev Pathol. 2010;13:24-31.
- Chauhan SC, Ebeling MC, Maher DM, et al. MUC13 mucin augments pancreatic tumorigenesis. Mol Cancer Ther. 2012;11:24-33.
- Chauhan SC, Vannatta K, Ebeling MC, et al. Expression and functions of transmembrane mucin MUC13 in ovarian cancer. Cancer Res. 2009;69:765-74.
- Gupta BK, Maher DM, Ebeling MC, et al. Increased expression and aberrant localization of mucin 13 in metastatic colon cancer. J Histochem Cytochem. 2012;60:822-31.
- Copin MC, Devisme L, Buisine MP, et al. From normal respiratory mucosa to epidermoid carcinoma: expression of human mucin genes. Int J Cancer. 2000;86:162-8.
- Finkbeiner WE, Zlock LT, Morikawa M, et al. Cystic fibrosis and the relationship between mucin and chloride secretion by cultures of human airway gland mucous cells. Am J Physiol Lung Cell Mol Physiol. 2011;301:402-14.
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245-78.
- McAuley JL, Linden SK, Png CW, et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest. 2007;117:2313-24.
- Ravi A, Merlin D, Sitaraman SV. Quality is as important as the quantity: role of mucin glycosylation on intestinal barrier function. Gastroenterology. 2007;133:2065-67.
- Hollingsworth MA, Swanson BJ. Swanson, Mucins in cancer: Protection and control of the cell surface. Nat Rev Cancer. 2004;4:45-60.
- Xu H, Inagaki Y, Seyama Y, et al. Expression of KL-6/MUC1 in pancreatic cancer tissues and its potential involvement in tumor metastasis. Oncol Rep. 2011;26:371-6.
- Rachagani S, Macha MA, Ponnusamy MP, et al. MUC4 potentiates invasion and metastasis of pancreatic cancer cells through stabilization of fibroblast growth factor receptor 1. Carcinogenesis. 2012;33:1953-64.
- Rachagani S, Torres MP, Moniaux N, et al. Current status of mucins in the diagnosis and therapy of cancer. Biofactors. 2009;35(6):509-27.
- Andrianifahanana M, Moniaux N, Schmied BM, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: A potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033-40.
- Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochem Biophys Acta. 2006;1765:189-222.
- Bombardieri E, Seregni E, Lombardo C, Cantoni A, Bogni A, Botti C, Sfreddo L, Cataldo I. Mucin gene expression in lung cancer tissues. Int J Biol Markers 1994; 9: 262-3.
- Rasoul-Rockenschaub S, Zielinski CC, Kubista E, et al. Diagnostic value of mucin-like carcinoma-associated antigen (MCA) in breast cancer. Eur J Cancer Clin Oncol. 1989;25:1067-72.
- Premaratne P, Welén K, Damber JE, et al. O-glycosylation of MUC1 mucin in prostate cancer and the effects of its expression on tumor growth in a prostate cancer xenograft model. Tumour Biol. 2011;32:203-13.
- Rachagani S, Torres MP, Kumar S, et al. Mucin (Muc) expression during pancreatic cancer progression in spontaneous mouse model: potential implications for diagnosis and therapy. J Hematol Oncol. 2012;5:68.
- Morioka K. Mucin-producing pancreatic cancer. Nihon Geka Gakkai Zasshi. 1991;92:765-70.
- Ponnusamy MP, Lakshmanan I, Jain M, et al. MUC4 mucin-induced epithelial to mesenchymal transition: A novel mechanism for metastasis of human ovarian cancer cells. Oncogene. 2010;29:5741-54.
- Woods MO, Younghusband HB, Parfrey PS, et al.The genetic basis of colorectal cancer in a population-based incident cohort with a high rate of familial disease. Gut. 2010;59:1369-77.
- Williams SJ, Wreschner DH, Tran M, et al. MUC13, a novel human cell surface mucin expressed by epithelial and haemopoietic cells. J Biol Chem. 2001;276:18327-36.
- Maher DM, Gupta BK, Nagata S, et al. Mucin 13: Structure, function, and potential roles in cancer pathogenesis. Mol Cancer Res. 2011;9:531-7.
- Corrales RM1, Galarreta DJ, Herreras JM, et al. Normal human conjunctival epithelium expresses MUC13, MUC15, MUC16 and MUC17 mucin genes. Arch Soc Esp Oftalmol. 2003;78:375-381.
- Kerschner JE. Mucin gene expression in human middle ear epithelium. Laryngoscope. 2007;117:1666-76.
- Seo JT, Lee JS, Jun JH, et al. Expression of mucin genes in the human testis and its relationship to spermatogenesis. Yonsei Med J. 2005;46:667-72.
- Imai K, Itoh F. Abnormalities in colonic mucin may contribute to the persistence of colonic mucosal inflammation. J Gastroenterol. 2000;35:71-2.
- Guo X, Pace RG, Stonebraker JR, et al. Mucin variable number tandem repeat polymorphisms and severity of cystic fibrosis lung disease: Significant association with MUC5AC. PLoS One. 2011;6:e25452.
- Chuang SC, His E, Lee KT. Mucin genes in gallstone disease. Clin Chem Acta. 2012;413:1466-71.
- Sheng YH, Triyana S, Wang R, et al. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 2013;6:557-68.
- Packer LM, Williams SJ, Callaghan S, et al. Expression of the cell surface mucin gene family in adenocarcinomas. Int J Oncol. 2004;25:1119-26.
- Walsh MD, Young JP, Leggett BA, et al.The MUC13 cell surface mucin is highly expressed by human colorectal carcinomas. Hum Pathol. 2007;38:883-92.
- Shimamura T, Ito H, Shibahara J, et al. Overexpression of MUC13 is associated with intestinal-type gastric cancer. Cancer Sci. 2005;96:265-73.
- Shemirani N, Osipov V, Kolker A, Khampang P, Kerschner JE.Expression of mucin (MUC) genes in mucoepidermoid carcinoma. Laryngoscope. 2011;121:167-70.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, CA Cancer J Clin. 2016;66:7-30.
- Bruyère E, Jonckheere N, Frénois F, et al. The MUC4 membrane-bound mucin regulates esophageal cancer cell proliferation and migration properties: Implication for S100A4 protein. Biochem Biophys Res Commun. 2011;413:325-9.
- Streppel MM, Vincent A, Mukherjee R, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;43:1755-63.
- Zheng DJ, Cooke DT. A survival comparison of mucin-producing adenocarcinoma of the esophagus to conventional adenocarcinoma after esophagectomy. Am Surg. 2013;79:49-53.
- Wang H, Shen L, Lin Y, et al. The expression and prognostic significance of Mucin 13 and Mucin 20 in esophageal squamous cell carcinoma. J Cancer Res Ther. 2015;11:74-9.
- Shen LY, Wang H, Dong B, et al. Possible prediction of the response of esophageal squamous cell carcinoma to neoadjuvant chemotherapy based on gene expression profiling. Oncotarget. 2016;7:4531-41.
- Tamura Y, Higashi M, Kitamoto S, et al. MUC4 and MUC1 expression in adenocarcinoma of the stomach correlates with vessel invasion and lymph node metastasis: An immunohistochemical study of early gastric cancer. PLoS One. 2012;7:e49251.
- Lee HS, Lee HK, Kim HS, et al. MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: Their roles as prognostic indicators. Cancer 2001;92:1427-34.
- Wang JY, Chang CT, Hsieh JS, et al. Role of MUC1 and MUC5AC expressions as prognostic indicators in gastric carcinomas. J Surg Oncol. 2003;83:253-60.
- Wang RQ, Fang DC. Alterations of MUC1 and MUC3 expression in gastric carcinoma: relevance to patient clinicopathological features. J Clin Pathol. 2003;56:378-84.
- Lee HJ, Nam KT, Park HS, et al. Gene expression profiling of metaplastic lineages identifies CDH17 as a prognostic marker in early stage gastric cancer. Gastroenterology. 2010;139:213-25.
- Suh YS, Lee HJ, Jung EJ, et al. The combined expression of metaplasia biomarkers predicts the prognosis of gastric cancer. Ann Surg Oncol. 2012;19:1240-9.
- Liu C, Smet A, Blaecher C, et al. Gastric de novo Muc13 expression and spasmolytic polypeptide-expressing metaplasia during Helicobacter heilmannii infection. Infect Immun. 2014;82:3227-39.
- Subrungruanga I, Thawornkunob C, Chawalitchewinkoon-Petmitrc P, et al.Gene expression profiling of intrahepatic cholangiocarcinoma. Asian Pac J Cancer Prev. 2013;14:557-63.
- Khan S, Sikander M, Ebeling MC, et al. MUC13 interaction with receptor tyrosine kinase HER2 drives pancreatic ductal adenocarcinoma progression. Oncogene 2016;218.
- Khan S, Ebeling MC, Zaman MS, et al.MicroRNA-145 targets MUC13 and suppresses growth and invasion of pancreatic cancer. Oncotarget. 2014;5:7599-609.
- Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. Cancer J Clin. 2013;63:11-30.
- Lauriola M, Ugolini G, Rosati G, et al. Identification by a digital gene expression displayer (DGED) and test by RT-PCR analysis of new mRNA candidate markers for colorectal cancer in peripheral blood. Int J Oncol. 2010;37:519-25.
- Gupta BK, Maher DM, Ebeling MC, et al. Functions and regulation of MUC13 mucin in colon cancer cells. J Gastroenterol. 2014;49:1378-91.
- Moehle C, Ackermann N, Langmann T, et al. Aberrant intestinal expression and allelic variants of mucin genes associated with inflammatory bowel disease. J Mol Med (Berl). 2006;84:1055-66.
- Begue B, Verdier J, Rieux-Laucat F, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am J Gastroenterol. 2011;106:1544-55.
- Sheng YH, Triyana S, Wang R, et al. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 2012;6:577-68.
- Sheng YH, Lourie R, Lindén SK, et al. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut. 2011;60:1661-70.
- Carr JC, Sherman SK, Wang D, et al. Overexpression of membrane proteins in primary and metastatic gastrointestinal neuroendocrine tumors. Ann Surg Oncol. 2013;3:739-746.