Case Report - Research and Reports in Gynecology and Obstetrics (2017) Research and Reports in Gynecology and Obstetrics (Special Issue 2-2017)
Ovarian function preservation of low grade endometrial stromal sarcoma at stage II: A case report and review of literature.
Yinjuan Chang1, Juanqing Li1, Yongshen Fan2, Yibing Lan1, Haiyan Shi1, Jianhong Zhou1*1Women’s Hospital School of Medicine, Zhejiang University, Hangzhou, Zhejiang, P.R. China
2Department of General Surgery, Zhejiang Hospital, Hangzhou, Zhejiang, P.R. China
- *Corresponding Author:
- Jianhong Zhou
Women’s Hospital School of Medicine
Zhejiang University
Hangzhou
Zhejiang
P.R. China
Tel: +86-18806516679
E-mail: zhoujh1117@zju.edu.cn
Accepted Date: February 09, 2018
Citation: Chang Y, Li J, Fan Y, et al. Ovarian function preservation of low grade endometrial stromal sarcoma at stage II: A case report and review of literature. Res Rep Gynaecol Obstet. 2018;2(1):5-8
Abstract
Endometrial stromal sarcoma (ESS) is a rare form of uterine cancer. Low grade endometrial stromal sarcoma (LG-ESS) is a special type of ESS, has an indolent clinical course, and it is associated with a favorable prognosis. It is very challenging to diagnose LG-ESS preoperative. The standard treatment for LG-ESS is surgical, with a total hysterectomy and bilateral salpingo-oophorectomy (BSO). There is no consensus regarding the role of adjuvant treatment, including chemotherapy, radiation, and hormonal treatment. Here we report a case of a 27-yearold female with LG-ESS at stage II who underwent total hysterectomy and salpingectomy. Medroxyprogesterone acetate and tamoxifen were then administered to inhibit tumor recurrence. Ovarian function was preserved for this young patient, with no sign of recurrence at the 11-months follow-up.
Keywords
Endometrial stromal sarcoma, Hormonal treatment, Treatment strategies
Introduction
Uterine sarcoma is a rare form of uterine cancer and accounts for 3-7% of all uterine cancer diagnoses in the United States [1]. According to the National Comprehensive Cancer Network (NCCN) 2015 classification, uterine sarcomas are categorized as uterine leiomyosarcoma (uLMS), endometrial stromal sarcoma (ESS), and undifferentiated uterine sarcoma (UUS) [2]. There are two types of endometrial stromal tumors (ESS): Low grade ESS (LG-ESS), and high grade endometrial sarcomas (HG-ESS). The classification is based on morphology, mitotic activity, cellularity, and the presence of necrosis. LG-ESS has an indolent clinical course, and is associated with a favorable prognosis in comparison with HG-ESS [3]. Standard treatment for LG-ESS is surgical, with a total hysterectomy with bilateral salpingo-oophorectomy (BSO). There is no consensus regarding the role of adjuvant treatment, including chemotherapy, radiation, and hormonal treatment.
We report here a case of LG-ESS at stage II who was treated by total hysterectomy and salpingectomy, and subsequent adjuvant treatment. Ovarian function was preserved for this young patient, with no sign of recurrence at the 11-months follow-up.
Case Presentation
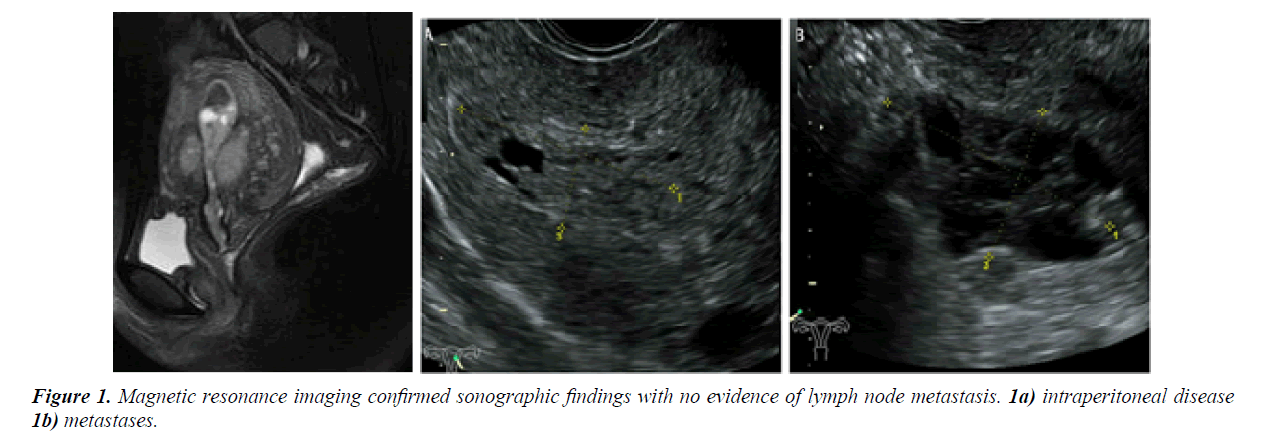
A 27-year-old nulligravid girl was admitted to the women hospital of medicine Zhejiang University (Hangzhou, Zhejiang, China) with symptom of abnormal vaginal bleeding for several days. Pelvic examination displayed a nontender mass from the lateral wall of the uterus without pain, and no adnexal mass was palpatable. A transvaginal ultrasonography demonstrated a heterogeneous echo (3.0 × 2.3 cm) in uterine cavity, and a honeycomb echo (4.2 × 4.6 cm) in the lateral wall of the uterus (Figure 1). The bilateral annex area was normal. Magnetic resonance imaging confirmed sonographic findings with no evidence of lymph node metastasis, intraperitoneal disease and ormetastases.
The patient underwent hysteroscopy for two times due to prolapsed of a neoplasm from the endometrial cavity from 2013 to 2015 in other hospitals, and pathology revealed endometrial polyps with mesenchymal cells proliferation activity and uterine tumors resembling ovarian sex cord tumors (UTROSCT) respectively. After her admission into our hospital this time, we hold a pathological consultation. The pathological consultation opinions were uterine tumors resembling ovarian sex cord tumors (UTROSCT) and low-grade endometrial stromal sarcoma with adenoid, sexual cord like differentiation respectively.
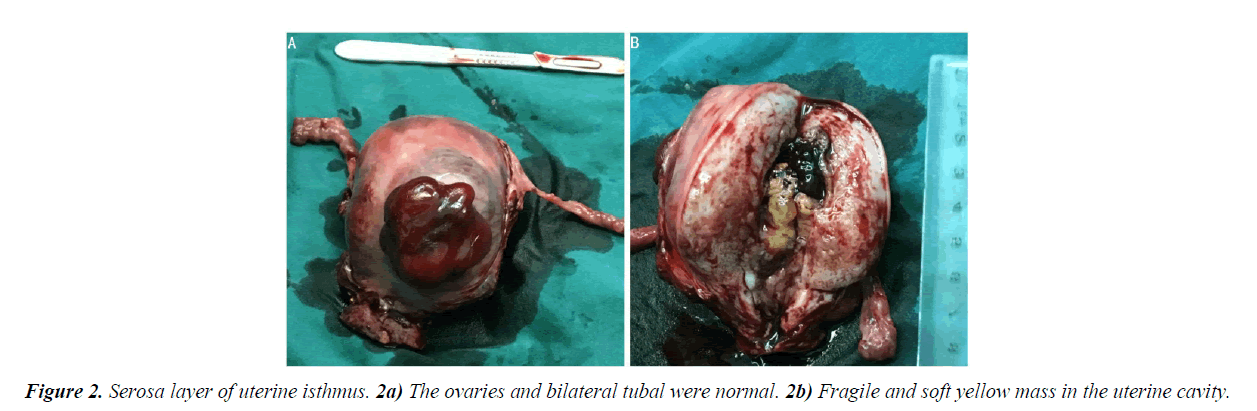
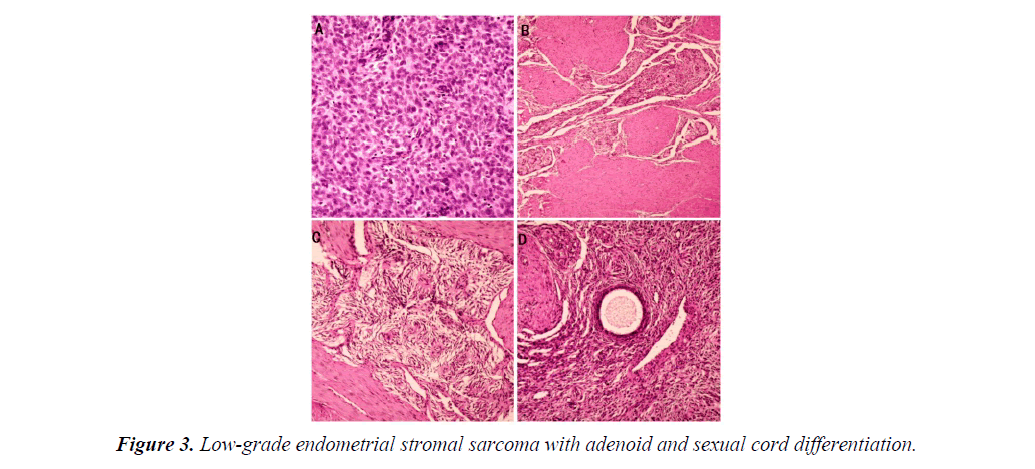
An exploratory laparotomy was performed after five days of adjuvant examinations. Upon surgical exploration, no effusion was found in the pelvic cavity. The uterus was of normal size. A mass was found on the anterior wall of the uterus, it was fragile, with dark red appearance, with unclear margins. A similar mass was also seen in the serosa layer of uterine isthmus (Figure 2A). The ovaries and bilateral tubal were normal. There was no enlargement of pelvic lymph nodes and no invasion of other pelvic tissue. Frozen section revealed endometrial stromal sarcoma. As the patient's family was expected to preserving ovarian function, so total hysterectomy and salpingectomy were performed. Gross specimen indicated that there was a fragile and soft yellow mass in the uterine cavity (Figure 2B). Final pathology revealed a low-grade endometrial stromal sarcoma with adenoid and sexual cord differentiation (Figure 3).
Sarcoma cells were involving the internal orifice of the uterus and serosal layer. The immunohistochemistry assay showed the following results: CD10(+), CK(+), EMA(+), WT1(+), cyclinD1(+++), ER(+++), PR(+++), Ki-67(++), Caldesmon(-), SMA(-), Desmin(-), Calretinin(-), Inhibin(-).
Post-operatively, the patient was administered 250 mg medroxyprogesterone acetate and 20mg tamoxifen daily to inhibit tumor recurrence. Sequential clinical examinations and radiographical studies have been used in post-operative surveillance, and at the time of writing, the patient is in a good condition.
Discussion
ESSs are rare tumors of mesenchymal origin [4]. ESSs are classified based on cell morphology and mitotic count into either low-grade (LG-ESS) or high-grade (HG-ESS) tumors. Women with LG-ESS are younger than women with other uterine sarcomas, and a median age of the patients with LGESS was between 45 and 57 years old [5]. In the current case, the patient is as young as 27 years old. It is very challenging to diagnose LG-ESS preoperative. The symptoms can be vague and nonspecific and may comprise lower abdominal or pelvic pain, abdominal distension, progressive menorrhagia and abnormal vaginal bleeding [6]. In this case, the young patient presented with abnormal vaginal bleeding and recurrence of neoplasm in the endometrial cavity.
Accurate diagnosis of LG-ESS is difficult even when specimens are examined by HE staining and histopathology. The differential diagnoses for LG-ESS include smooth muscle tumours, adenomyosis with sparse glands and uterine tumour resembling ovarian sex cord tumour (UTROSCT) [7]. In the present case, the patient underwent hysteroscopy in 2013 and 2015 respectively; the pathology revealed UTROSCT and low-grade endometrial stromal sarcoma with adenoid, sexual cord like differentiation, and it was very difficult to identify the LG-ESS and UTROSCT. It is helpful to using appropriate immunostains in differential diagnosis. The sex cord differentiation in a LG-ESS is also not well characterized in the literature. However, we found the rare type of LG-ESS with sex cord differentiation in this case.
Standard treatment for LG-ESS is surgical, with a total hysterectomy with bilateral salpingo-oophorectomy (BSO). Several authors have proposed that local resection of lowgrade endometrial stromal sarcoma may be a viable option for patients desiring future fertility, considering the good prognosis of low-grade endometrial stromal sarcoma [7]. And some authors recommend hysterectomy with salpingooophorectomy when childbearing was completed [8]. In this case, because the patient underwent recurrence of the disease and there were lesions in the uterine cavity and serosal layer, so total hysterectomy and salpingectomy were performed after carefully consideration.
The role of BSO in treating LG-ESS is debated. BSO has become a mandatory part of standard surgical management because estrogen may act as a trophic agent for LG-ESS, and there were concerns of higher relapse rates if the ovaries were retained. Although this issue has less importance in perimenopausal and postmenopausal women, bilateral oophorectomy deserves particular consideration in young women. However, several researchers indicated that ovarian preservation was safe and did not affect the outcomes of patients with early stage LG-ESS [9]. In our opinion, ovary-sparing procedures might be considered in younger patient at early stage. As the patient was only 27-yearsold and bilateral ovaries were no invasion, so bilateral ovaries were reserved to improve her quality of life. And closed followup is needed for her after the surgery.
The status of lymphadenectomy (LAD) in the management and staging of LG-ESS is still controversial, and there are no clear surgical guidelines. Goff et al. did not find nodal metastases after retroperitoneal staging/sampling with LG-ESS who underwent lymphadenectomy at the first surgery [10]. On the other hand, some findings suggest that the incidence of lymph node involvement in low-grade endometrial stromal sarcoma is higher than expected [11]. More extensive sampling of lymph nodes in a larger number of patients may allow a better understanding of the frequency and prognostic significance of these metastases. In this case, pelvic systematic lymphadenectomy was not performed, and further follow-up is necessary for her.
There is also no consensus regarding the role of adjuvant treatment, including chemotherapy, radiation, and hormonal treatment. The data on the response of LG-ESS to chemotherapy were scarce because the date from the era where high-grade and low-grade ESS were pooled. 80% of LG-ESS expresses estrogen receptor (ER) alpha and progesterone receptor (PgR), providing an opportunity for adjuvant endocrine therapy for both prevention and treatment of recurrences. Progestins or aromatase inhibitors can be considered [12]. The major antiproliferative actions include downregulation of estrogen receptor, increased estrogen metabolism and inhibition of estrogen-induced growth factors, thus inhibiting the growth-stimulating effects of estrogen on estrogen receptor-positive cells. The optimal duration of adjuvant hormonal therapy for ESS is undetermined. In the present study, the young patient with stage II LG-ESS showed no signs of recurrence following ovaries conservative treatment and high-dose progestin treatment.
Conclusion
In conclusion, LG-ESS has an indolent clinical course, and is associated with a favorable prognosis; ovary-sparing procedures might be considered in younger patient. However, no standard treatment strategy has yet been established. Future investigation should include more study participants and focus on the response to treatment and prognosis.
References
- D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131-9.
- Koh WJ, Greer BE, Abu-Rustum NR, et al. Uterine Sarcoma, Version 1.2016: Featured Updates to the NCCN Guidelines. J Nat Compr Canc Netw. 2015;13(11):1321-31.
- Gadducci A, Cosio S, Romanini A, et al. The management of patients with uterine sarcoma: a debated clinical challenge. Crit Rev Oncol Hematol. 2008;65(2):129-42.
- Chew I, Oliva E. Endometrial stromal sarcomas: a review of potential prognostic factors. Adv Anat Pathol. 2010;17(2):113-21.
- Landréat V, Paillocher N, Catala L, et al. Low-grade endometrial stromal sarcoma of the uterus: review of 10 cases. Anticancer Res. 2008;28(50):2869-74.
- Ali RH, Rouzbahman M. Endometrial stromal tumours revisited: an update based on the 2014 WHO classification. J Clin Pathol. 2015;68:325-32.
- Stadsvold JL, Molpus KL, Baker JJ, et al. Conservative management of a myxoid endometrial stromal sarcoma in a 16-year-old nulliparous woman. Gynecol Oncol. 2005;99(1):243-5.
- Koskas M, Morice P, Yazbeck C, et al. Conservative management of low-grade endometrial stromal sarcoma followed by pregnancy and severe recurrence. Anticancer Res. 2009;29(10):4147-50.
- Shah JP, Bryant CS, Kumar S, et al. Lymphadenectomy and ovarian preservation in low-grade endometrial stromal sarcoma. Obstet Gynecol. 2008;112(5):1102-8.
- Goff BA, Rice LW, Fleischhacker D, et al. Uterine leiomyosarcoma and endometrial stromal sarcoma: lymph node metastases and sites of recurrence. Gynecol Oncol. 1993;50(1):105-9.
- Riopel J, Plante M, Renaud MC, et al. Lymph node metastases in low-grade endometrial stromal sarcoma. Gynecol Oncol. 2005;96(2):402-6.
- Thanopoulou E, Aleksic A, Thway K, et al. Hormonal treatments in metastatic endometrial stromal sarcomas: the 10-year experience of the sarcoma unit of Royal Marsden Hospital. Clin Sarcoma Res. 2015;5(1):8.