- Biomedical Research (2006) Volume 17, Issue 2
otal antioxidant activity in Parkinson's Disease
Usha Adiga, D’souza J, Kousalya R, Rao G M*, Nandini M and D’souza VDepartment of Biochemistry, Kasturba Medical College, Mangalore, India
- *Corresponding Author:
- Gayathri M Rao
Associate Professor
Dept. of Biochemistry, K.M.C., Mangalore, India
Tel: 0091-8242211767
E-mail: gayatrimrao@yahoo.com
Accepted date: May 16, 2006
Abstract
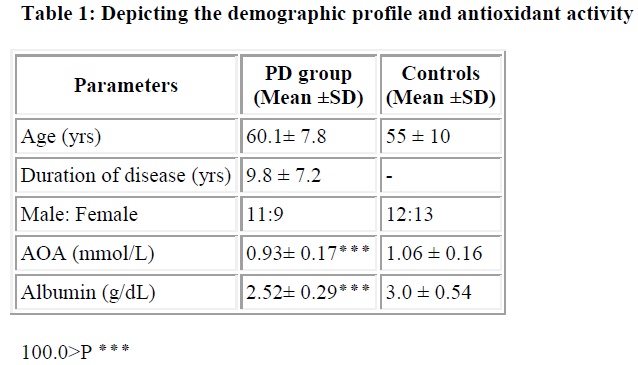
Parkinson’s disease (PD) is one of the major progressive neurological disorders, characterized by the loss of dopaminergic neurons in pars compacta of the substantia nigra. The causes for this is the interactions between external toxins (which arise from environmental, dietary and life style factors) and internal toxins arising from normal metabolism, genetic and epigenetic (mitochondria, membranes and proteins) components. Oxidative stress is one of the intermediary risk factors that could initiate and promote degeneration of neurons. Even though oxidative stress in brain is an important factor in the neuropathology of PD, yet the role of systemic oxidative stress is inconclusive. In the present study, we estimated the total antioxidant activity (AOA) and albumin levels in twenty PD patients who were on levodopa, and in age matched twenty-five normal con-trols. We observed a significant reduction in total antioxidant activity in PD patients with a mean value of 0.93 ± 0.17 mmol/l as compared to 1.06 ± 0.16 mmol/l in controls (P < 0.001). Albumin levels were also significantly reduced in PD patients, with a mean of 2.52 ± 0.29 g/dl as compared to 3.0 ± 0.54 g/dl in controls (P < 0.001). It could be concluded from our study that the diminution in total antioxidant activity could be due to the reduction of radical trapping capacity of naturally occurring antioxidant albumin, which in turn could be due to reduced dietary supplementation or defective absorption of amino acids. Supplemen-tation of multiple antioxidants may slow down the progression of the disease.
Keywords
Parkinson’s disease, neurological disorders, substantia nigra, toxins, oxidative stress, neuropathology
Introduction
Oxidative stress is implicated in neuronal loss associated with neurodegeneration in Parkinson’s disease (PD). Dopaminergic nigrostrial neurons, the predominant cells lost in PD are believed to be highly prone to oxidative damage. This is due to the propensity of dopamine to autooxidize and thereby produce an elevated levels of H2O2 which reacts with transition metal iron (Fe+3) and forms the highly reactive and cytotoxic hydroxyl radicals (OH-) which are known to damage lipids, proteins and DNA [1-3]. Iron is frequently associated with neurode-gerative process [4,5]. The basal ganglia in particular, the globus pallidus and substantia nigra contain high concentration of iron which contributes to free radical produc-tion. Accumulation of iron in substantia nigra contributes to the cell death by enhancing lipid peroxidation, as judged by raised levels of both malondialdehyde and lipid hydroperoxides [6]. There are reports that antioxidant enzymes in plasma are found to be more active but to the best of our knowledge there is no conclusive evidence on the total systemic antioxidant activity in PD patients [7,8]. Hence we planned a study and correlated the systemic antioxidant activity and albumin levels in patients of PD.
Subjects and Methods
Twenty PD patients who were on treatment with anti-Parkinson’s drugs (Levodopa) and twenty-five healthy age matched controls were selected in this study after tak-ing the written informed consent. The individuals in the control group were nonsmokers nonalcoholics, free from diseases. The conventional criteria for the diagnosis of Parkinson’s disease is the presence of at least one of the following cardinal features, i.e akinesia, rigidity, resting tremors [9].
Estimation of total antioxidant activity
The AOA was estimated by spectrophotometric method of Koracevic et al [10]. Two milliliter of blood samples were collected in EDTA bottles. Plasma was separated immediately by centrifugation and AOA is measured. This method is based on the principle that the standard-ized solution of iron EDTA complex reacts with hydrogen peroxide by a Fenton type of reaction, leading to the formation of hydroxyl radicals. This reactive oxygen spe-cies degrades benzoate, resulting in the release of TBARS. Antioxidants from the added plasma cause the suppression of production of TBARS. The reaction is measured spectrophotometrically at 532nm. The inhibit-tion of the colour developed is defined as AOA [11].
Estimation of plasma albumin
Albumin level was estimated colorimetrically [12]. The reaction between albumin and bromocresol green produces change in colour that is proportional to the albumin concentration.
Results were analyzed using student’s paired ‘t’ test.
Results
We observed a significant reduction in total antioxidant activity in PD patients with a mean value of 0.93 ± 0.17 mmol/L as compared to 1.06 ± 0.16 mmol/L in controls (P<0.001). Albumin levels were also significantly reduced in PD patients, with a mean of 2.52 ± 0.29 g/dL as compared to 3.0 ± 0.54 g/dL in controls (P<0.001).
Discussion
Decrease in AOA indicates disturbance in the antioxidant defence system of the body. This could be due to decrease in individual antioxidants. There are controversial reports regarding various individual antioxidants in PD. Some studies reported an increased antioxidant enzymes such as SOD, glutathione peroxidase [7,8] whereas others have showed a reduction in antioxidant enzymes viz SOD and catalase [13]. Reduced levels of antioxidants such as glu-tathione and uric acid [14], vitamin C & vitamin E [15,16] have also been reported. Several others have reported an insignificant change in erythrocyte SOD, catalase, glutathoine peroxidase and antioxidant vitamins such as vitamin C and vitamin E in parkinson’s patients [17-19]. However, no report is so far available regarding the total systemic antioxidant status in parkinson’s patients. In the present study, we have measured total antioxidant activity, a dynamic equilibrium that is influenced by the interactions between each antioxidative constistuent. The significant reduction in total antioxidant activity observed in this study could be due to greater reduction of some antioxidants which is not compensated by an increase in some other antioxidants.
Ten to fifty percent of the total radical trapping capacity of antioxidants in plasma is contributed by plasma proteins. Albumin, a major plasma protein, which contributes significantly to AOA is decreased in PD patients when compared to normal controls. Decreased albumin levels can be attributed to poor eating or difficulty in swallow-ing or difficulty in moving and getting the food or due to depression i.e. as a consequence of disease progression.
Decreased albumin levels can be due to reduced absorption and utilization of amino acids. Phenylalnine tyrosine, and tryptophan can all be blocked from absorption by levodopa, thereby becoming deficient [20-22]. This could be due to the competition for the carriers across the intes-tinal wall. There are reports that suggest a significant re-duction in plasma protein containing SH groups and albumin levels in parkinson’s patient [23,24]. The net total antioxidant activity may be more important rather than individual antioxidant activity. The reduced total antioxi-dant activity may contribute to the imbalance between prooxidants and antioxidants resulting in oxidative stress and leading to neuronal damage.
The result of the present study strongly suggests that the reduction in AOA seen in PD subjects is due to the decreased plasma albumin. It may be attributed to the inefficient absorption of amino acids. Thus it may be con-cluded that supplementation protein rich diet may in-crease albumin levels and hence increase AOA, provided given after the drug is absorbed. Supplementation of multiple antioxidants along with protein adjusted diet will maintain a balanced antioxidant status and may slow down the progression of the disease. Further studies should be conducted to observe whether AOA can be used as a marker of improvement.
Acknowledgement
The authors acknowledge Dr. I G Bhat and Dr. Shanker for their support and subjects for their cooperation.
References
- Nils H, Joseph G,Jean CS, Enrique C. The metabolism of tyrosine by MAO A/MAO B causes oxidative damage to mitochondrial damage. Arc Biochem Biophys 1996; 335: 295-304.
- Cohen G. Oxyradical toxicity in catecholamine neu-rons. Neurotoxicology 1984; 5: 77-82.
- Cohen G. Monoaminooxidase. Hydrogen peroxide and parkinson’s disease. Adv Neurol 1987; 45: 119-125.
- Gerlach M, Ben SD, Riederer P Youdm MBM. Altered brain metabolism of iron as a cause of neurodegenerative disease? J Neurochem. 1994; 63: 793-807.
- Perry G, Taddeo MA, Peterson RB, Castellani RJ, Harris PLR, Sicdlak SL, Cash AD, Liu Q, Nunamura A, Atwood CS, Smith MA. Adventitiously bound redox active iron and copper are at the centre of oxidative damage in Alzheimer disease. Biometals 2000; 6:70-81.
- Riederer P, Rausch WD,Schmidt B et al. Biochemical fundamentals of Parkinsons’s disease. Mt SinaiJ.Med 1988; 55: 21-28
- TV Ilic, Jovanovic M, Jovicicand A, Tomovic M. Oxidative stress indicators are elevated in de novo Parkinson’s disease patients. Funct Neurology 1999; 14: 141-147.
- J Karla J, Rajput AH, Mantha SV, Prasad K. Serum antioxidant enzyme activity in Parkinson’s disease. Mol Cell.Biochem 1992; 10: 165-168 .
- Andrew J Hughes, Yoav BS, Susan E D, Andrew JL. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: A clinicopathological study. Neurology. 1992; 42: 1142-1146.
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J. Clin Pathol 2001; 54: 356-361.
- Gutteridge JMC, Maidt L, Poyer L. Superoxide dismutase and Fenton chemistry. Biochem J. 1990; 269: 169-174.
- Doumasa BT, Watson WA, Biggs HG. Albumin stan-dards and the measurement of serum albumin with bromocresol green. Clin Chim Acta 1971; 31: 87-96.
- Bostantjopoulou S, Kyriazis G, Katsarou Z, Kiosseog-lou G, Kazis A, Mentenopoulos G. Superoxidedismutase activity in early and advanced Parkinson’s dis-ease. Funct Neurol 1997; 12:63-1268.
- Ihara Y, Chuda M, Kuroda S, Hayabara T. Hydroxyl radical and Superoxide dismutase in patients with Parkinson’s disease: Relationship to clinical data. J.Neurol Sci 1999; 170: 90- 95.
- Sudha K, Rao A, Rao S, Rao A. Free radical toxicity and antioxidants in Parkinson’s disease. Neurol India 2003; 51: 60- 62.
- Mc De Rijik , Breteler MMB, den Breejien J H. Dietary antioxidants and Parkinson’s disease. Arch.Neurol, 1997; 54: 762-775.
- Fernandez- calle P, Jimenez-jimenez FJ, Molina J.A Arjona Serum levels of ascorbic acid (Vitamin C) in patients with PD. J Neuro Sci 1993;118: 25-28.
- Jimenez-jimenez FJ, Molina Arjona JA, Fernandez- calle P, Vazquez A, Cabrera VF, Catalan MJ, Gracia-Albea E, Bermejo F, Codoceo R. Serum levels of beta carotene and other carotenoids in Parkinson’s diseae. Neuro Sci Lett 1993 ; 157: 103-106.
- King D, Playfer JR, Roberts NB. Concentration of vitamins A, C, E in elderly patients with Parkinson’s disease. Post grad med J. 1992; 68: 634-637.
- Heller B, Fischer E, Maetin R. Therapeutic action of d- Phenylalanine in PD. Arzneim Forsch. 1976; 26: 577-579.
- Yamaguchi T, Nagatsu T. Effects of tyrosine administration on serum biopterin in normal controls and patients with Parkinson’s disease. Science. 1983; 219: 75-77.
- Lehmann J. Tryptophan malabsorption in levodopa treated parkisonian patients. Acta Med Scand. 1973; 194: 181-189.
- Carsten B, Sonke A, Anatol K, Tobias MB, Sinje S, Matthias O, Hans-Joerg S, Ulrike B. Plasma and CSF marker of oxidative stress are increased in parkinson’s disease and influenced by antiparkinsonian mediation. Neurobiology of disease. 2004; 15: 160-170.
- Ravikumar A, Deepadevi KV, Arun P, Manoj Kumar V, Kurup PA. Tryptophan and tyrosine catabolic pattern in neuropsychiatric disorders. Neurology India 2000; 48: 231- 238.