Research Article - Current Pediatric Research (2022) Volume 26, Issue 10
Nutrition in preschoolers with emphasis on prebiotics: a review
Sailajanandan Parida1,2, Suresh Kumar S3, Preethi Rahul4*, Amit Khandeparkar4
1Department of Neonatal Health and Human Nutrition, Asian Institute of Public Health, Bhubaneswar, Odisha, India
2Department of Pediatrics and Head Neonatology, SCB Medical College, Cuttack, Odisha, India
3Department of Neonatology and Director, Pragna Hospitals, Hyderabad, Telangana, India
4Nutricia International Private Limited, Mumbai, (Danone India)
- *Corresponding Author:
- Preethi Rahul
Nutricia International Private Limited,
Mumbai, (Danone India)
E-mail: Preethi.RAHUL@danone.com
Received: 02 October, 2022, Manuscript No. AAJCP-22-75103; Editor assigned: 05 October, 2022, PreQC No. AAJCP-22-75103(PQ); Reviewed: 12 October, 2022, QC No. AAJCP-22-75103; Revised: 19 October, 2022, Manuscript No. AAJCP-22-75103(R); Published: 27 October, 2022, DOI:10.35841/0971-9032.26.9.1185-1189.
Abstract
Child under-nutrition increases the risk of childhood mortality and poor cognitive development and over-nutrition is associated with increased risk of various non-communicable diseases. Despite substantial economic growth in India over most recent decades, chronic malnutrition (stunting) in children less than five years of age (preschoolers) remains alarmingly high, with 35.5% of children stunted in the country. The triple burden of malnutrition continues to be a significant public health challenge in most countries. While food supplementation with essential nutrients can help mitigate the micronutrient deficiencies in preschoolers, inefficient nutrient absorption in the gut can in turn contribute to micronutrient deficiencies. Prebiotic is defined as a substrate that is selectively utilized by host microorganisms conferring a health benefit. With availability of advanced knowledge regarding the gut microbiota, it is now proven that prebiotics help in nutrient absorption. Additionally, prebiotics also help in developing a strong immune system. The strategy of food supplementation with micronutrients and prebiotics is thus beneficial in reducing micronutrient deficiencies in preschoolers. It is seen that in India, milk remains major part of diet in preschoolers; while milk alone is not nutrient-rich, studies have now shown that supplementing milk with other specific nutrients like calcium and zinc can support growth. Studies have now shown that combining prebiotics along with essential micronutrients like iron, zinc and calcium may support growth and development in preschoolers.
Keywords
Gut microbiome, Immunity, Micronutrients, Nutrients, Oligosaccharides, Prebiotics, Pre-schoolers.
Introduction
Good nutrition is pivotal to a child’s physical growth and cognitive development. In children, especially preschoolers (3-6 years of age), growth in height and weight is steady, with a similar amount of growth each year until the next major growth spurt in early adolescence [1]. On the other hand, different organs grow at different rates while the brain exhibits dramatic biological development and roughly quadruples in weight before the age of six [2], when it has acquired approximately 90% of its adult volume [3-6]. Thus, nutrition becomes the cornerstone of the healthy growth and development of preschoolers.
Some of the common concerns seen in preschool children in India include delayed growth, underweight and overweight; feeding delays, oral-motor problems, medication/nutrient interactions, gut health concerns (constipation and diarrhea), altered energy and nutrients needs (inborn errors of metabolism), various infections, poor or excessive appetite and specialized diets like vegan diets that deprive the child of right amounts of nutrition.
These concerns affect the child in several ways. Poor nutritional intake and poor appetite is the prime reason for these children to be underweight. It can also be due to food deprivation. Another important concern of under-nutrition, especially in developing countries, is malnutrition which conjures up a picture of a grossly underweight or wasted child.
Malnutrition refers to deficiencies, excesses, or imbalances in a person’s intake of energy and/or nutrients. The term malnutrition includes under-nutrition, micronutrient-related malnutrition and overweight/obesity. Under-nutrition includes wasting (low weight-for-height), stunting (low height-for-age) and underweight (low weight-for-age). Micronutrient-related malnutrition includes micronutrient deficiencies (a lack of important vitamins and minerals) or micronutrient excess. Overweight conditions include obesity and diet-related non-communicable diseases (such as heart disease, stroke, diabetes and some cancers) [7].
UNICEF in its report of 2006 mentioned the causes of childhood malnutrition as insufficient diet, frequent infections, poor breastfeeding practices, delayed introduction of complementary foods and inadequate protein in the diet. Such a child is markedly underweight for his/her age, as well as for his/her height. Long-term malnutrition leads to impaired growth in children often seen as stunting. It is estimated that stunting affects >30% of children aged <5 years in low- and middle-income countries [8]. In India alone, 35.5% of preschool children are stunted [9]. Further, state-level estimates depict a substantially higher burden among poor children from states like Uttar Pradesh, Bihar, Haryana, Jharkhand, and Madhya Pradesh. Morbidity and mortality are highest among those most severely malnourished [10].
Another result of malnutrition is obesity, where a child consumes more calories than required and often from poor sources i.e. foods which are not nutrient dense. Childhood obesity is increasing at an alarming pace in India. The percentage of overweight children under five years of age has increased from 2.1% in 2015 to 3.4% in 2021 [9]. Obesity adversely impacts the child’s physical health, social, and emotional well-being, academic performance, and self-esteem. Furthermore, it also gives rise to adult diseases in youth, like high blood pressure, Type 2 Diabetes Mellitus (T2DM) and heart disease [11]. Moreover, high sugar foods coupled with poor oral hygiene are most likely to lead to dental caries in children [12].
Micronutrient deficiency (also known as “hidden hunger” and hereafter denoted as “MiND”) occurs when the intake or absorption of essential vitamins and minerals falls below levels necessary for growth and development in children [13]. Micronutrient malnutrition, commonly called “hidden hunger” because it is less obvious than regular hunger. Since it is harder to identify visually, hidden hunger gets far less attention than it warrants. Hidden hunger is particularly detrimental to young children [14,15].
Micronutrients are required in small quantities and are responsible for vital functions in the human body. Nutritional deficiencies in one or more nutrients during these crucial years of development not just impedes a child’s nutrition and development in the short term, but also negatively affects the cognitive abilities and results in poor weight gain along with a deceleration of linear growth (growth faltering). This results in stunting, which is an important indicator of linear growth retardation, and results in reduced educational attainment, reduced adult economic productivity, and increased risk for non-communicable illnesses (like diabetes) in adulthood [16,17].
Major causes of micronutrient deficiencies range from inadequate micronutrient consumption mostly owing to a lack of dietary diversity to poor nutrient absorption. This review focusses on burden of micronutrient deficiencies amongst preschoolers in India, and how food supplementation with prebiotics and essential nutrients can help combat these micronutrient deficiencies in preschoolers.
Early-life gut microbial compositionRecent advances in genomic sequencing technologies have resulted in a great appreciation for the gut microbiome and its role in physiological processes that affect health. The gastrointestinal tract mediates digestion and absorption of dietary constituents, facilitates communication with peripheral tissues via various signaling molecules produced by the gut bacteria [18]. Prebiotics play an important role in improving the nutrient absorption in the gastrointestinal tract and thereby exert a health-promoting effect.
The infant’s gut undergoes important developmental stages that are entirely dependent the colonization, with microorganisms, beginning at birth. In a matter of days, however, the intestinal lumen turns anaerobic, allowing for strict anaerobes, such as Bifidobacterium, Clostridium, and Bacteroides to colonize. By 2–3 years of age, the microbiota composition consists mainly of Bacteroidaceae, Lachnospiraceae, and Ruminococcaceae, which then remains stable into adulthood. Diet driven alteration of the intestinal microbiota can feed back into host metabolism and immunity, with differing consequences depending on the composition and metabolic potential of the colonizing microbes. In both malnutrition and obesity, the interrelationship between the microbiota, metabolism, and immunity plays an important role in determining outcomes of severity of diseases. The microbiota in children under 3 years of age fluctuates substantially and is more impressionable to environmental factors than the adult microbiota [19].
The first 4 years of life are a critical window where long-term developmental patterns of Body Mass Index (BMI) are established and a critical period for microbiota maturation. A study in Canadian children showed that infants in the rapid growth trajectory were less likely to have been breastfed and gained less microbiota diversity in the first year of life [20].
Prevalence of micronutrient deficiencies amongst preschoolers in IndiaCritical nutrients required by preschoolers, include protein, long chain polyunsaturated fats, iron, copper, zinc, choline and vitamins, with protein, iron and zinc being common to all development stages [21,22]. India has the highest prevalence of clinical and subclinical vitamin A deficiency among South Asian countries; 62% of preschool children were reported to be deficient in vitamin A. About 57% of preschoolers and their mothers have subclinical Vitamin A deficiency. Anemia is a major public health problem in India as well as globally affecting nearly a third of the global population. Recent survey data suggests that in India, 41% of preschoolers have anemia and 32% of preschoolers have iron deficiency. Furthermore, among preschoolers, 22% have mild anemia, 18% have moderate anemia and 1% have severe anemia. Additionally, 21% of preschoolers were both anaemic and iron deficient, 11% were iron deficient but not anaemic, and 18% were anaemic but not iron deficient [23].
Another common micronutrient deficiency is iodine deficiency; according to World Health Organization estimates, goiter rates among school-age children exceed 5% in 130 countries, putting 2,225,000 people at risk [24]. Approximately 6.6 million children are born mentally impaired every year in India, which reduces intellectual capacity by 15% across India due to iodine deficiency; 200,000 babies are born every year with neural tube defects due to folic acid deficiency [25]. Nearly 23% of preschoolers in India are found to have a folate deficiency. Zinc deficiency is characterized by growth retardation, loss of appetite, and impaired immune function.is estimated to be responsible for about 800,000 deaths annually from diarrhea, pneumonia, and malaria in children less than 5 years of age [26]. In India, zinc deficiency in preschoolers is found to be 19%. Among children aged 1–4 years, zinc deficiency is more common in rural areas (20%) vs. urban areas (16%) and in the poorest households (24%) vs. the richest households (16%).
Consequences of micronutrient deficienciesThere is now increasing evidence suggesting the role of early malnutrition in increasing the risk of numerous chronic diseases in adulthood [27]. In India, 69% of preschoolers are reported to have a fever or acute respiratory infections [9]. Respiratory, mostly upper respiratory infections and gastrointestinal illnesses were reported to be the most common childhood diseases attributed to malnutrition in children living in semi-urban slums of a southern Indian city [28]. Primary vitamin A deficiency could be attributed to prolonged deprivation of vitamin A-rich foods and is further depleted by diarrhea, measles, and respiratory infection [29].
Iron deficiency is the primary cause of anemia, although vitamin A deficiency, folate deficiency, malaria, and HIV also result in anemia. It has negative effects on work capacity [30] and on motor and cognitive development in children and adolescents [31,32]. About half of anemia cases worldwide are estimated to be due to iron deficiency [33]. Iron deficiency without anemia also has been associated with negative impacts on cognitive development in children [34].
In India, iron intake is lower than required since Indian meals are predominantly plant based (rice, pulses and vegetables) with high phytic acid levels. The absorption of iron from these diets is likely to be poor. Therefore, dietary practices followed in most Indian homes may be a contributing factor for iron deficiency among preschoolers [35].
Iron is depleted primarily through blood loss, including from parasitic infections such as schistosomiasis and hookworm. A study conducted in children of rural, urban and urban slums of India suggested that anemia was positively associated with vitamin intake in children (P<0.001); worm infestation p<0.001) and iron intake (p<0.001). The same study showed a higher prevalence of anemia in boys compared to girls (p=0.019) [36]. Another major cause of iron deficiency is inadequate iron absorption. Iron absorption is tightly regulated in the intestines, depending on the iron status of the individual, the type of iron, and other nutritional factors. Once iron is absorbed, it is well conserved.
Iodine is necessary for thyroid hormones that regulate growth, development, and metabolism and is essential to prevent goiter and cretinism. Inadequate intake can result in impaired intellectual development and physical growth. Iodine deficiency impairments range from fetal loss, stillbirth, congenital anomalies, to hearing impairment; a vast majority of deficient individuals experience mild mental retardation.
This decrease in mental ability and work capacity may have significant economic consequences. Zinc deficiency results from inadequate intake and, to some extent, increased losses. Research done in India also demonstrated that zinc deficiency was significantly associated with anemia and wasting (P<0.002) [37].
Multiple Micronutrient Deficiencies (MNDs) are reported more frequently in children below 5 years of age and adversely impact growth and development. Low food diversity, poor diet intakes, and low bioavailability combined with high physiological demands and frequent infections contribute to the etiology of MNDs. Assessment of micronutrient inadequacies at the population level and adjustment of nutrient levels in fortified foods and/or supplements needs to be considered [38].
Single micronutrient deficiency occurs rarely, while concurrent micronutrient deficiencies may increase vulnerability to infection, and the resulting inflammatory response also affects the interpretation of several micronutrient status indicators. To combat this, dietary supplementation with different proportions of requisite micronutrients is done. It should, however, be noted that multivitamins and mineral supplements are not intended to replace food but should complement current diets and help bridge the gap between inadequacy and sufficiency. Another important thing to consider while selecting vitamin and mineral supplements for optimum nutrient absorption is the bioavailability of these nutrients.
It is now a well-established fact that vitamin C is necessary to absorb iron from plant-based food items (non-heme iron) [39,40]. Vitamin C helps to convert the ferric form of iron from plants into a ferrous form that the body can absorb easily. Another classic example to explain how increased bioavailability helps in better nutrient absorption is Vitamin K2.
Both Vitamin K1 and Vitamin K2 are readily absorbed within 2 hours of ingestion by the gut; however, postprandial serum concentrations of K2 (MK-7) are reported to be 10-fold higher than K1. Long chain derivatives of vitamin K2 have a longer half-life in circulation in comparison to K1 and thus are available for longer in circulation to be absorbed by extrahepatic tissue [41]. Vitamin K2, required in calcium metabolism, improves bone density and reduces the risk of bone fractures.
Strategies for improving nutrient status in preschoolersAccording to UNICEF, common strategies to combat hidden hunger include food supplementation, fortification, improving food quality, and using ready-to-use supplements (like sprinkles, etc.). Direct supplementation of vulnerable sub-populations with micronutrients, usually through a primary healthcare system or healthcare delivery system such as an immunization program, has been shown to be effective and very cost-effective for young children for vitamin A and zinc [42].
Fortification is another safe, effective, and affordable tool to enhance the nutritional value of staple food products such as wheat, maize (corn), rice, vegetable oil, and sugar. While food fortification is a widely preferred tool used in mass nutrition programs, it is challenging to improve the nutritional requirement of “picky eaters,” especially preschoolers. One simplest example is milk which is a part of the everyday diet of most kids. Milk is deficient in several nutrients, and hence milk fortification with nutrients is necessary. Supplementation of milk with nutrients is associated with increases in weight gain, linear and skeletal growth.
Prospective clinical studies have shown that milk protein with calcium supplementation can increase the acquisition of bone mass during childhood, adolescence, and early adulthood [43,44]. A study on healthy 2.5 year-old Danish children concluded that milk intake has a stimulating effect on the growth of a child [45]. Furthermore, another study in China revealed that children consuming dairy products at least once daily had a 28% lower risk of stunting than children without dairy intake. Thus, dairy consumption is an effective and feasible nutritional intervention for improving linear growth in preschool children [46].
Improving absorption of nutrients and bioavailabilityConsidering the diverse dietary landscape where micronutrients come from a variety of foods, with a sizeable proportion of vitamins and minerals secured through fortified foods and supplements, it is important to assess total nutrient exposure in an individual. A potential risk of interactions between micronutrient absorption and bioavailability needs to be considered in any supplementation or fortification strategy. At levels of essential micronutrients present in foods, most micronutrients appear to utilize specific absorptive mechanisms and not be vulnerable to interactions. In aqueous solutions and at higher intake levels, competition between elements with similar chemical characteristics and uptake by non-regulated processes can take place. This is evident from studies demonstrating the role of ascorbic acid and phytates in the modulation of iron bioavailability and how phytates may impact zinc bioavailability [47]. When nutrient absorption is concerned, it is important to consider the form of nutrient that is easily absorbed by the body.
Role of prebiotics in nutrient absorptionA prebiotic is defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [48]. Fructo-Oligosaccharides (FOS) and Galacto-oligosaccharides (GOS) are the most researched prebiotics [49]. Fermentation of prebiotics by gut microbiota produces lactic acid, butyric acid, and propionic acid, collectively called Short-Chain Fatty Acids (SCFAs). These products can have positive effects on human health, like improving gut health and boosting the immune system. Since gut health is known to affect distant organs, prebiotics has the potential to manage and prevent certain diseases. A study conducted in rural African preschool children showed that abnormal gut integrity is associated with reduced linear growth [50].
The World Health Organization’s (WHO) multi-center growth reference study defined three anthropometric (physical) parameters (weight-for-age, height-for-age, and weight-for-height Z scores) to describe normal early childhood growth and nutritional status [51]. Children with Severe Acute Malnutrition (SAM) have a persistent developmental abnormality affecting their gut microbial “organ” that is not durably repaired with existing therapy. Alterations in the normal postnatal development of the gut microbiota may trigger marked impairments in brain development and lead to persistent disorders of cognition. Evidence exists for a causal relationship between the gut microbiota and SAM but also highlights the importance of diet-by-microbiota interactions in disease pathogenesis [52].
The gut microbiota of children who are undernourished is usually immature, that is, more similar to microbiota of younger children than to that of age-matched healthy controls. Thus, microbiota immaturity is causally related to under nutrition [53]. There is a link between postnatal growth, gut microbiota and identified microbial factors that could improve growth. Certain microbial species can counteract the negative effects of under nutrition and raise the possibility that the microbiota could be used as a therapeutic intervention to restore healthy growth [54].
Stunting, defined as a height-for-age Z score equal to or lower than -2, is associated with increased childhood mortality, cognitive impairment, and chronic diseases. A study in Peruvian children showed that stunting was preceded by an increase in markers of enterocyte turnover and differences in the fecal micro biota and was associated with increasing levels of systemic inflammation markers [55]. Environmental Enteric Dysfunction (EED) and Systemic Inflammation (SI) are common in developing countries and may cause stunting. A study on preschoolers showed that the effects of EED on constrained weight gain might have consequences for later linear growth or for other health and development outcomes [56].
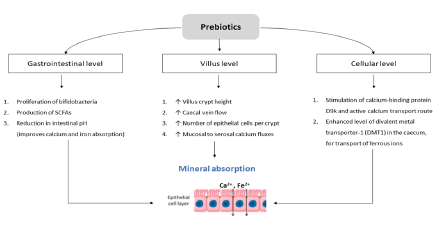
Absorption of minerals in the gastrointestinal tract is influenced by several factors; including. It has been hypothesized that prebiotics that results in pH reduction, a pronounced osmotic effect, stimulation of the exchange of protons, an increased level of butyrate and calbindin (calcium-binding protein), and an enlarged surface area in the colon for increasing the absorption of minerals and trace elements [57,58].
There is growing evidence that prebiotics may be used to improve the absorption of micronutrients (such as calcium and iron) from ingested foods. Generally, in the human intestine, the enzymes that hydrolyze the polymer bonds of prebiotics are lacking, so they can resist small-intestinal digestion and reach the colon intact, where they undergo fermentation by beneficial bacteria, such as Lactobacilli and Bifidobacteria. The consumption of prebiotics is known to change intestinal microbiota diversity and to increase the production of SCFAs like propionate and butyrate [59-61].
Although the exact action of prebiotics on microbial diversity in the colon is still under debate, an interesting study by Liu et al., carried out using healthy volunteers, found a significant decrease in butyrate production levels (attributed to high lactic acid levels) and an increase in Bifidobacteria levels, after the administration of FOS [62]. The mechanism of action of prebiotics is shown in Figure 1. Studies in both humans and animals showed positive effects of prebiotics, especially the non-digestible oligosaccharides on mineral metabolism, bone composition, and bone architecture [63-67]. The use of FOS to improve the absorption of minerals and trace elements seems to be beneficial with evidence corroborating both in human and animal studies [68-72]. Another recent review concluded that inulin and GOS increased the iron absorption in duodenum and proximal colon and hence when combined with iron fortificants can better tackle iron deficiency anemia [73]. Inulin has been shown to increase the absorption of other minerals as well, such as magnesium and iron [74,75].
Several FOS with different degree of polymerization stimulated magnesium absorption and retention, but magnesium retention was stimulated more significantly with oligofructose while calcium retention was stimulated only with a blend of oligofructose and an inulin with a high degree of polymerization than with 2 different types of inulin. In adolescents 15 g/d of oligofructose stimulated calcium absorption, as did 8 g/d of a combination of oligofructose and inulin in girls n [76,77]. An observational study suggested that six‐month prebiotic feeding was associated with favorable outcomes in anthropometrics, appetite, gastrointestinal tolerance and safety in malnourished children [78]. A four month, controlled, double-blind trial in Kenyan infants showed that the abundance of Virulence and Toxin Genes (VTGs) of pathogens was significantly lower in the group receiving GOS (7.5g) iron-containing Micronutrient Powders (MNPs) compared with the control and iron groups (p<0.01). The addition of GOS mitigates most of the adverse effects of iron on the gut microbiome and morbidity in infants [79]. Prebiotic supplementation with fructans modified the composition of the intestinal microbiota and resulted in softer stools in preschool children (3-6 years) [80] (Table 1).
| Study | Prebiotic used | Study design | Population | N | Key findings |
|---|---|---|---|---|---|
| Van den Heuvel, et al. | Oligofructose | Randomized, double-blind, cross-over | Adolescent boys | 12 | An increase in true fractional calcium absorption (%) was found after consumption of oligofructose (mean difference ± SE of difference: 10.8 ± 5.6; P<0.05). 15gms/day |
| Oligofructose stimulated fractional calcium absorption in male adolescents. | |||||
| Griffin IJ, et al. | Non-digestible oligosaccharides | Randomized. Cross-over | girls | 59 | Calcium absorption was significantly higher in the group receiving the inulin + oligofructose mixture than in the placebo group v. P=0.01), but no significant difference was seen between the oligofructose group and the placebo group v. P=NS). Modest intakes of an inulin+oligofructose mixture increases calcium absorption in girls at or near menarche. |
| Paganini D, et al. | MNP only (control) vs. MNP+ iron 5mg (Fe group) vs. MNP+iron 5mg + GOS 7.5g (FeGOS group) | controlled, double-blind, randomized -controlled, double-blind trial | Infants (6.5-9.5 mo) | 155 | Anaemia decreased by ≈50% in the Fe and FeGOS groups (p<0.001). the abundance of virulence and toxin genes (VTGs) of all pathogens was significantly lower in the FeGOS group (p<0.01) |
| Whisner CM, et al. | Smoothie containing GOS (2.5 or 5 g) vs. control (0g) | Randomized, double-blind cross-over trial | Adolescent girls (age 10-13 years) | 31 | Significant improvements in Ca absorption were seen with both low and high doses of prebiotic (GOS) compared with the control (P< 0·02) |
| Drabińska N, et al. | oligofructose-enriched inulin 10g or placebo (maltodextrin) | Preliminary Randomized, Placebo-Controlled Nutritional Intervention Study | Children (4–17 years) with celiac disease on a strict gluten-free diet | 34 | Children receiving prebiotic had a 42% increase in 25(OH)D (p<0.05) and a 19% increase in vitamin E (p<0.05) |
| Yap KV, et al. | Inulin (0.75g/d, 1.00g/d, and 1.25g/d) | Dose comparison, Randomized study | Formula-fed infants | 36 | Infants on 1/g prebiotic (inulin) had significantly higher apparent absorption, per cent apparent retention and net retention of iron |
| All three dose of inulin lead to increase in per cent apparent retention and net retention of magnesium. | |||||
| Infants on 0.75g/day inulin had improved zinc absorption and retention (All P<0.05) |
Table 1. Role of prebiotics in nutrient absorption in humans.
Several preclinical studies further support these findings that prebiotics stimulate the absorption of calcium, magnesium, and zinc in short-term experiments and improved bone mineral content [81-84]. Furthermore, the FOS-inulin mixture is found to increase the number of goblet cells and the thickness and composition of the colonic epithelial mucus layer. This composition shifts toward more acidic mucins, predominantly sulfomucins, i.e., changes that indicate a more stabilized mucosa. All these effects are regarded as beneficial for the health maintenance of the gut because they improve its absorptive function [85]. Absorption of calcium and magnesium was stimulated in animals receiving inulin or resistant starch. However, the effect on calcium absorption was more significant if both inulin and resistant starch were given combined [86].
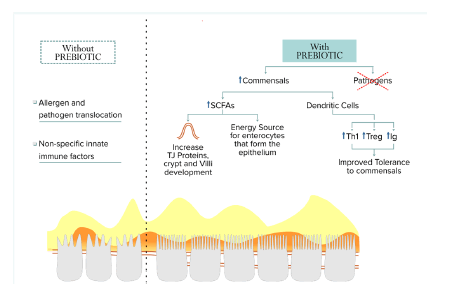
Prebiotics in immune responsePrebiotics modulate the immune system by multiple mechanisms (Figure 2). Firstly, prebiotics bind to pathogens and prevent adhesion to the epithelial surface, thus preventing subsequent infection. Secondly, prebiotics promote populations of commensal microbes, which outcompete pathogens for resources, thus reducing infections. Furthermore, prebiotics modulate gut health by improving barrier integrity and increasing the number of Short-Chain Fatty Acids (SCFAs) producing commensal microbes that increase mucus, Tight Junction (TJ) proteins, crypt, and villi development while also serving as an energy source [57,87].
Nutrients studies have shown that specific prebiotics have the potential to reduce the risk of infectious episodes and the development of allergic symptoms in infants [88]. Positive functional characteristics of prebiotics have been reported in selective fermentation, modulation of gut pH, fecal bulking, the prevention of gut colonization by pathogens, and the control of putrefactive bacteria, thus reducing the host’s exposure to toxic metabolites [89].
Supplementing kindergarten children during a cold season with a prebiotic inulin-type fructans product with shorter and longer fructan chains has been shown to reduce febrile episodes requiring medical attention and to lower the incidence of sinusitis [90] (Table 2). A stabilized flora contributes to prevent gastrointestinal infections and oxidative damage to the enterocytes. Another study demonstrated a reduced risk of infection following consumption of growing-up milk supplemented with scGOS/lcFOS/n-3 long-chain polyunsaturated fatty acids in young children [91] (Table 2).
| Study | Prebiotic used | Population | N | Results |
|---|---|---|---|---|
| Kadim M, et al. | Supplementation of Zinc, Glutamine, Fiber, and Prebiotics | Children 1-3 years old | N=200 | A transitory decrease in Fecal Calprotectin (FC) was observed after 6 months in the subgroup with normal FC levels, who were fed the test formula (p=0.012). |
| Lohner S, et al. | Fructans 6 g/d or control (maltodextrin) | Children 3-5 years old | N=219 | Increase in abundance of bifidobacterium (P <0.001) and that of Lactobacillus (P=0.014) were 19.9% and 7.8% higher in fructans group as compared to control group at week 24. |
| Significantly softer stools within the normal range in the prebiotic group from week 12 onwards. | ||||
| The incidence of febrile episodes requiring medical attention (P=0.04] and that of sinusitis (P=0.03) were significantly lower in the prebiotic group. | ||||
| Soldi S, et al. | 6 g/day prebiotic inulin-type fructans or maltodextrin. | Children 3-6 years old | N=258 | Relative abundance of bifidobacterium was significantly higher in the prebiotic group compared to control group (effect found for all three enterotypes). |
| Children of the prebiotic group receiving antibiotic treatment displayed significantly higher levels of bifidobacterium than children receiving the placebo control. | ||||
| Chatchatee P, et al. | GUM with scGOS/lcFOS/LCPUFAs | Children, ages 11 to 29 months | N=767 | Children in the active group compared with the control group had a decreased risk of developing at least 1 infection (77% vs. 83%, RR 0.93, P=0.03). |
| Reduction (P=0.07) in the total number of infections in the active group (69% vs. 77%, RR 0.89, P=0.004, post hoc). | ||||
| More infectious episodes were observed in the cow's milk group, when compared with both GUM groups (92% vs. 80%, RR1.15). |
Table 2. Role of prebiotics in immunity.
Role of nutrient supplementation on growth and developmentResearch done so far has emphasized the benefit of iron supplementation on cognitive performance among primary-school–aged children, especially on IQ among children with anemia; additionally, iron may also improve growth [92]. On the other hand, while findings from previous studies of infant and child supplementation with zinc in developed and developing countries have ranged from no effect to significant reductions in growth retardation and stunting, several recent studies have confirmed that supplementation with zinc-plus (multivitamin approach) can have greater impact on growth development of children as these nutrients are closely linked together [93-97]. Fecal Calprotectin (FC) and alpha-1-antitrypsin (α1AT) are markers of mucosal integrity in toddlers. Increased levels of FC may indicate inflammation causing decreased mucosal integrity leading to reduced GI function and nutrient malabsorption in diarrheal diseases.
Prebiotics are known to stimulate the development of Bifidobacteria in the gastrointestinal microbiome, which may strengthen the mucosal resistance toward gastrointestinal infection and zinc supplementation is known to increase the immunocompetence that affects the clearance of diarrhea-causing pathogens [98]. Together they might reasonably be considered significant in reducing the intestinal inflammation in apparently healthy-looking kids both with low baseline FC levels. Daily micronutrient and prebiotic supplementation could thus benefit growth and development (cognitive and immune system) at the individual level especially in settings where these deficiencies are prevalent at population level [99].
Conclusion
There exists the problem of triple burden of malnutrition with coexistence of overnutrition, undernutrition and micronutrient deficiencies, all of which equally increase the risk of various health problems. Undernutrition increases the risk of childhood mortality, stunting, low BMI and poor cognitive development. Food supplementation with essential nutrients can help mitigate the micronutrient deficiencies in children especially preschoolers. However, providing only nutrient rich foods to children may not completely reverse the micronutrient deficiency as nutrient absorption and bioavailability can be some major confounding factors. With advancing knowledge in the gut microbiota, it is now proven that prebiotics help in nutrient absorption. Additionally, prebiotics also help in developing a strong immune system. The strategy of food supplementation with micronutrients and prebiotics is thus beneficial in reducing micronutrient deficiencies.
Abbreviations
- α1 AT: Alpha 1-antitrypsin
- Body Mass Index: BMI
- Environmental Enteric Dysfunction: EED
- Fecal Calprotectin: FC
- Fructooligosaccharide: FOS
- Galactooligosaccharide: GOS
- Growing Up Milk: GUM
- Human Immunodeficiency Virus: HIV
- Micronutrient Deficiency: MiND
- Micronutrient Powder: MNPs
- Multiple Micronutrient Deficiencies- MND
- Severe Acute Malnutrition: SAM
- Short-Chain Fatty Acids: SCFA
- Systematic Inflammation: SI
- The United Nations Children's Fund: UNICEF
- Virulence and Toxic Genes: VIGs
- World Health Organization: WHO
The authors acknowledge Dr. Nalinikant Panigrahy (Consultant Neonatologist, Rainbow Children’s Hospital, Hyderabad) for his valuable input to this article. We would also like to acknowledge Intellimed Healthcare Solutions LLP for medical writing support.
Statements and Declaration
FundingThe Article Processing Charges (APC) were paid by Nutricia International Pvt. Ltd. (Danone India).
Conflict of interestsPreethi Rahul and Amit Khandeparkar are the employees of Nutricia International Private Limited (Danone India)
Author contributionAll authors contributed to the content of the manuscript. All authors read and approved the final manuscript.
AuthorshipAll named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Ethics approvalThis article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availabilityData sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
- https://www.msdmanuals.com/en-in/home/children-s-health-issues/growth-and-development/physical-growth-of-infants-and-children
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child 1973; 48: 757–767.
- Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 2000; 216: 672–682.
- Iwasaki N, Hamano K, Okada Y, et al. Volumetric quantification of brain development using MRI. Neuroradiology 1997; 39: 841–846.
- Kennedy DN, Makris N, Herbert MR, et al. Basic principles of MRI and morphometry studies of human brain development. Developmental Science 2002; 5: 268–278.
- https://www.researchgate.net/publication/278357693_Gradients_and_boundaries_limits_of_modularity_and_its_influence_on_the_isocortex_-_Commentary
- https://www.who.int/news-room/fact-sheets/detail/malnutrition
- Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013; 382 (9890): 427–51.
- http://rchiips.org/nfhs/
- https://www.academia.edu/37037440/Comparative_Quantification_of_Health_Risks_Global_and_Regional_Burden_of_Disease_Attributable_to_Selected_Major_Risk_Factors
- Sahoo K, Sahoo B, Choudhury AK, et al. Childhood obesity: Causes and consequences. J Family Med Prim Care 2015; 4(2): 187.
- Gupta P, Gupta N, Pawar AP, et al. Role of sugar and sugar substitutes in dental caries: A review. ISRN Dent 2013; 2013: 519421.
- Ritchie H, Reay DS, Higgins P. Quantifying, projecting, and addressing India's hidden hunger. Front Sustain Food Syst 2018: 11.
- https://www.intechopen.com/online-first/81420
- Lowe NM. The global challenge of hidden hunger: Perspectives from the field. Proceedings of the Nutrition Society. 2021; 80(3): 283-9.
- Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: Findings from five birth cohort studies. Lancet 2013; 382: 525–534.
- Perez-Escamilla R. Post-1000 day’s growth trajectories and child cognitive development in low- and middle-income countries. Am J Clin Nutr 2013; 98: 1375–1376.
- Whisner CM, Castillo LF. Prebiotics, bone and mineral metabolism. Calcified Tissue International 2018; 102(4): 443-79.
- Arrieta MC, Stiemsma LT, Amenyogbe N, et al. The intestinal microbiome in early life: health and disease. Front Immunol 2014; 5: 427.
- Reyna ME, Petersen C, Dai DL, et al. Longitudinal body mass index trajectories at preschool age: Children with rapid growth have differential composition of the gut microbiota in the first year of life. Int J Obes (Lond) pp.1-8.
- Cusick SE, Georgie MK. The role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J Pediatr 2016; 175: 16–21.
- https://www.unicef-irc.org/article/958-the-first-1000-days-of-life-the-brains-window-of-opportunity.html
- https://nhm.gov.in/WriteReadData/l892s/1405796031571201348.pdf
- https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-019-7505-7
- Kotecha PV. Micronutrient Malnutrition in India: Let Us Say "No" to it Now. Indian J Community Med 2008; 33(1): 9-10.
- https://pubmed.ncbi.nlm.nih.gov/21250337/
- Caballero B. Early Nutrition and Risk of Disease in the Adult. Public Health Nutr 2001; 4(6A): 1335–36.
- Sarkar R, Sivarathinaswamy P, Thangaraj B. et al. Burden of childhood diseases and malnutrition in a semi-urban slum in southern India. BMC Public Health 2013; 13: 87.
- Eduardo Villamor, Wafaie W Fawzi. Vitamin A supplementation: Implications for morbidity and mortality in children. J Infec Diseases 2000; 182(1): S122–S133.
- Haas JD, Brownlie T IV. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J Nutr 2001; 131: 676S-690S.
- https://www.cpsp.cps.ca/uploads/publications/RA-iron-defi ciency-anemia.pdf
- Grantham-McGregor S, Ani C. A review of studies on the effect of iron defi ciency on cognitive development in children. J Nutr 2001; 131: 649S-668S.
- WHO 2007a World Health Organization. Conclusions and recommendations of the WHO consultation on prevention and control of iron defi ciency in infants and young children in malaria-endemic areas. Food Nutr Bull 2007; 28: S621–S627.
- https://www.who.int/publications/i/item/9789241513067
- https://journals.lww.com/ijmr/Fulltext/2013/37020/Effect_of_iron_status_on_iron_absorption_in.11.aspx
- Onyeneho, NG, Ozumba BC, et al. Determinants of Childhood Anemia in India. Sci Rep 2019; 9(1): 16540
[Crossref] [Google Scholar] [Indexed]
- Dhingra U, Hiremath G, Menon VP, et al. Zinc deficiency: Descriptive epidemiology and morbidity among preschool children in peri-urban population in Delhi, India. J Health Popul Nutr 2009; 27(5): 632-9.
[Crossref] [Google Scholar] [Indexed]
- Madhari RS, Boddula S, Ravindranadh P, et al. High dietary micronutrient inadequacy in peri?urban school children from a district in South India: Potential for staple food fortification and nutrient supplementation. Matern Child Nutr 2020; 16: e13065.
[Crossref] [Google Scholar] [Indexed]
- https://pubmed.ncbi.nlm.nih.gov/3700141/#:~:text=The%20results%20indicate%20that%20ascorbic,is%20desirable%20for%20optimum%20effect.
- Lynch SR, Cook JD. Interaction of vitamin C and iron. Ann NY Acad Sci 1980 Dec 1; 355(1): 32-44.
[Crossref] [Google Scholar] [Indexed]
- Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 2000; 30(6): 298-307.
[Crossref] [Google Scholar] [Indexed]
- https://ncdalliance.org/sites/default/files/resource_files/Global%20Burden%20of%20Disease.pdf
- Vatanparast H, Bailey DA, Baxter-Jones AD, et al. The effects of dietary protein on bone mineral mass in young adults may be modulated by adolescent calcium intake. J Nutr 2007; 137(12): 2674–9.
[Crossref] [Google Scholar] [Indexed]
- Matkovic V, Goel PK, Badenhop-Stevens NE, et al. Calcium supplementation and bone mineral density in females from childhood to young adulthood:A randomized controlled trial. Am J Clin Nutr 2005; 81(1): 175–88.
[Crossref] [Google Scholar] [Indexed]
- Hoppe C, Rovenna Udam T, Lauritzen L, et al. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-y-old Danish children. Am J Clin Nutr 2004; 80(2): 447-52.
[Crossref] [Google Scholar] [Indexed]
- Duan Y, Pang X, Yang Z, et al. Association between dairy intake and linear growth in Chinese pre-school children. Nutrients 2020; 12(9): 2576.
[Crossref] [Google Scholar] [Indexed]
- Gibson RS, Raboy V, King JC. Implications of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nutrition reviews 2018; 76(11): 793-804.
[Crossref] [Google Scholar] [Indexed]
- Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the term and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017; 14: 1–12.
- Davani-Davari D, Negahdaripour M, Karimzadeh I, et al. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019; 8(3): 92.
[Crossref] [Google Scholar] [Indexed]
- Weisz AJ, Manary MJ, Stephenson K, et al. Abnormal gut integrity is associated with reduced linear growth in rural Malawian children. J Pediatr Gastroenterol Nutr 2012; 55(6): 747-50.
[Crossref] [Google Scholar] [Indexed]
- Subramanian S, Blanton LV, Frese SA, et al. Cultivating healthy growth and nutrition through the gut microbiota. Cell 2015; 161(1): 36-48.
[Crossref] [Google Scholar] [Indexed]
- Greenhill C. Growth impairment in undernourished children. Nat Rev Endocrinol 2016; 12(4): 186.
[Crossref] [Google Scholar] [Indexed]
- Du Toit A. Restoring healthy growth in infants. Nat Rev Microbiol 2016; 14(4): 191-192.
[Crossref] [Google Scholar] [Indexed]
- Zambruni M, Ochoa TJ, Somasunderam A, et al. Stunting is preceded by intestinal mucosal damage and microbiome changes and is associated with systemic inflammation in a cohort of Peruvian infants. Am J Trop Med Hyg 2019; 101(5): 1009-1017.
[Crossref] [Google Scholar] [Indexed]
- Campbell RK, Schulze KJ, Shaikh S, et al. Environmental enteric dysfunction and systemic inflammation predict reduced weight but not length gain in rural Bangladeshi children. Br J Nutr 2018; 119(4): 407-414.
[Crossref] [Google Scholar] [Indexed]
- McKeen S, Young W, Mullaney J, et al. Infant complementary feeding of prebiotics for the microbiome and immunity. Nutrients 2019; 11(2): 364.
[Crossref] [Google Scholar] [Indexed]
- Whisner CM, Castillo LF. Prebiotics, bone and mineral metabolism. Calcif Tissue Int 2018; 102(4): 443-79.
[Crossref] [Google Scholar] [Indexed]
- Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017; 8: 172–184.
[Crossref] [Google Scholar] [Indexed]
- Carlson JL, Erickson JM, Lloyd BB, et al. Health effects and sources of prebiotic dietary fiber. Curr Dev Nutr 2018; 2(3): nyz005
[Crossref] [Google Scholar] [Indexed]
- Carlson JL, Erickson JM, Hess JM, et al. Prebiotic dietary fiber and gut health: Comparing the in vitro fermentations of beta-glucan, inulin and xylooligosaccharide. Nutrients 2017; 9(12):1361.
[Crossref] [Google Scholar] [Indexed]
- Liu F, Li P, Chen M, et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate producing bacteria with adverse Glycemic metabolism in healthy young population. Sci Rep 2017; 7(1): 11789.
[Crossref] [Google Scholar] [Indexed]
- Scholz-Ahrens KE, Ade P, Marten B, et al. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J Nutr 2007; 137(Suppl 2): 838S–46S
[Crossref] [Google Scholar] [Indexed]
- Legette LL, Lee W, Martin BR, et al. Prebiotics enhance magnesium absorption and inulin-based fibers exert chronic effects on calcium utilization in a postmenopausal rodent model. J Food Sci 2012; 77(4): 88–94.
[Crossref] [Google Scholar] [Indexed]
- Martin BR, Braun MM, Wigertz K, et al. Fructo-oligosaccharides and calcium absorption and retention in adolescent girls. J Am Coll Nutr 2010; 29(4): 382–6.
[Crossref] [Google Scholar] [Indexed]
- Weaver CM, Martin BR, Nakatsu CH, et al. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J Agric Food Chem 2011; 59(12): 6501–6510.
[Crossref] [Google Scholar] [Indexed]
- Whisner CM, Martin BR, Schoterman MH, et al. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: A double-blind cross-over trial. Br J Nutr 2013; 110 (7): 1292–303.
[Crossref] [Google Scholar] [Indexed]
- Muijs T, Brouns F, Hendriks HFJ. Short-chain fructo-oligosaccharides improve magnesium absorption in adolescent girls with a low calcium intake. Nutr Res 2009; 29(4): 229-237.
[Crossref] [Google Scholar] [Indexed]
- Jakeman SA, Henry CN, Martin BR, et al. Soluble corn fiber increases bone calcium retention in postmenopausal women in a dose-dependent manner: A randomized crossover trial. Am J Clin Nutr 2016; 104(3): 837-843.
[Crossref] [Google Scholar] [Indexed]
- Wargovich MJ, Eng VW, Newmark HL. Calcium inhibits the damaging and compensatory proliferative effects of fatty acids on mouse colon epithelium. Canc Lett 1984; 23(3): 253-258.
[Crossref] [Google Scholar] [Indexed]
- Sukushima Y, Kumagai A. Prevention of osteoporosis by foods and dietary supplements. Chocolate malt drink MILO: Nutrition in children and calcium absorption. Clin Calcium 2006; 16(10): 1706-1713.
[Crossref] [Google Scholar] [Indexed]
- Weinborn V, Valenzuela C, Olivares M, et al. Prebiotics increase heme iron bioavailability and do not affect non-heme iron bioavailability in humans. Food Funct 2017; 8(5): 1994-1999.
[Crossref] [Google Scholar] [Indexed]
- Ahmad AMR, Ahmed W, Iqbal S, et al. Prebiotics and iron bioavailability? Unveiling the hidden association - A review, Trends Food Sci Technol 2021; 110: 584-590.
- Lopez HW. Fructooligosaccharides enhance mineral absorption and counteract the deleterious effects of phytic acid on mineral homeostasis in rats. J Nutr Biochem 2000; 11(10): 500-508.
[Crossref] [Google Scholar] [Indexed]
- Heuvel EGVD, Schaafsma G, Muys T, et al. Nondigestible oligosaccharides do not interfere with calcium and nonheme-iron absorption in young, healthy men. Am J Clin Nutr 1998; 67(3): 445-451.
[Crossref] [Google Scholar] [Indexed]
- Heuvel EGVD, Muys T, Dokkum VW, et al. Oligofructose stimulates calcium absorption in adolescents. Am J Clin Nutr 1999; 69(3): 544-548.
[Crossref] [Google Scholar] [Indexed]
- Griffin IJ, Davila PM, Abrams SA. Non-digestible oligosaccharides and calcium absorption in girls with adequate calcium intakes. Br J Nutr 2002; 87(S2): S187-S191.
[Crossref] [Google Scholar] [Indexed]
- Kansu A, Ugurcan DO, Arslan D, et al. High-fibre enteral feeding results in improved anthropometrics and favourable gastrointestinal tolerance in malnourished children with growth failure. Acta Paediatr 2018; 107(6): 1036-1042.
[Crossref] [Google Scholar] [Indexed]
- Paganini D, Uyoga MA, Kortman GA, et al. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: A randomised controlled study in Kenyan infants. Gut 2017; 66(11): 1956-1967.
[Crossref] [Google Scholar] [Indexed]
- Lohner S, Jakobik V, Mihalyi K, et al. Inulin-type fructan supplementation of 3- to 6-year-old children is associated with higher fecal bifidobacterium concentrations and fewer febrile episodes requiring medical attention. J Nutr 2018; 148(8): 1300-1308.
[Crossref] [Google Scholar] [Indexed]
- Scholz-Ahrens KE, Schaafsma G, van den Heuvel EGHM, et al. Effects of prebiotics on mineral metabolism. Am J Clin Nutr 2001; 73(2): 459s–64s.
[Crossref] [Google Scholar] [Indexed]
- Scholz-Ahrens KE, Schrezenmeir J. Inulin, oligofructose and mineral metabolism—experimental data and mechanism. Br J Nutr 2002; 87: Suppl2: S179–86.
[Crossref] [Google Scholar] [Indexed]
- Coudray C, Demigne C, Rayssiguier Y. Effects of dietary fibers on magnesium absorption in animals and humans. J Nutr 2003; 133: 1–4.
[Crossref] [Google Scholar] [Indexed]
- http://www.caister.com/backlist/ciim/v/v4/03.pdf
- Kleessen B, Hartmann L, Blaut M. Fructans in the diet cause alterations of intestinal mucosal architecture, released mucins and mucosa-associated bifidobacteria in gnotobiotic rats. Br J Nutr. 2003; 89: 597–606.
[Crossref] [Google Scholar] [Indexed]
- Younes H, Coudray C, Bellanger J, et al. Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesium balance in rats. Br J Nutr 2001; 86: 479–85.
[Crossref] [Google Scholar] [Indexed]
- Van der Beek CM, Dejong CHC, Troost FJ, et al. Role of of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr Rev 2017; 75: 286–305.
[Crossref] [Google Scholar] [Indexed]
- https://www.sciencedirect.com/science/article/abs/pii/S175646461300193X?via%3Dihub
- De Jesus Raposo MF, de Morais AM, de Morais RM. Emergent sources of prebiotics: seaweeds and microalgae. Mar Drugs 2016; 14: 27-29.
[Crossref] [Google Scholar] [Indexed]
- Soldi S, Vasileiadis S, Lohner S, et al. Prebiotic supplementation over a cold season and during antibiotic treatment specifically modulates the gut microbiota composition of 3-6 year-old children. Beneficial Microbes 2019; 10(3): 253-26.
[Crossref] [Google Scholar] [Indexed]
- Chatchatee P, Lee WS, Carrilho E, et al. Effects of growing-up milk supplemented with prebiotics and LCPUFAs on infections in young children. J Pediatr Gastroenterol Nutr 2014; 58(4): 428.
[Crossref] [Google Scholar] [Indexed]
- Low, Michael, Farrell, et al. Effects of daily iron supplementation in primary-school-aged children: Systematic review and meta-analysis of randomized controlled trials. CMAJ 2013; 185(17): E791-802.
[Crossref] [Google Scholar] [Indexed]
- Mozaffari-Khosravi H, Shakiba M, Eftekhari MH, et al. Effects of zinc supplementation on physical growth in 2–5-year-old children Biol. Trace Elem Res 2009; 128(2): 118-127.
[Crossref] [Google Scholar] [Indexed]
- Abrams SA. Nutritional rickets: An old disease returns. Nutr Rev 2002; 60 (4): 111-115.
[Crossref] [Google Scholar] [Indexed]
- Lawless W, Latham MC,Stephenson LS, et al. Iron supplementation improves appetite and growth in anemic Kenyan primary school children. J Nutr 1994; 124(5): 645-654.
[Crossref] [Google Scholar] [Indexed]
- T Clausen I. Dorup Micronutrients, minerals and growth control Bibl. Nutr Dieta 1998; 54:84-92.
[Crossref] [Google Scholar] [Indexed]
- Kadim M, Hegar B, Bardosono S, et al. Effect of supplementation of zinc, glutamine, fiber, and prebiotics in presumed healthy Indonesian children aged 1–3 year Pediatr Gastroenterol Hepatol Nutr 2020; 23(4): 388-396.
[Crossref] [Google Scholar] [Indexed]
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: Applications to improve child survival and reduce burden of disease. J Health Popul Nutr 2008; 26(3): 356-365.
[Crossref] [Google Scholar] [Indexed]
- Drabi?ska N, Krupa-Kozak U, Abramowicz P, et al. Beneficial effect of oligofructose-enriched inulin on vitamin D and E status in children with celiac disease on a long-term gluten-free diet: A preliminary randomized, placebo-controlled nutritional intervention study. Nutrients 2018; 10(11): 1768.
- Yap KW, Mohamed S, Yazid AM, et al. Dose?response effects of inulin on the faecal short?chain fatty acids content and mineral absorption of formula?fed infants. Nutrition and Food Science 2005.