Case Report - Journal of Oral Medicine and Surgery (2022) Volume 5, Issue 3
Nutrient-enriched microenvironments: An immunometabolic approach to the management of MRONJ -A case series.
Mark Tuffley*
Department of Oral and Maxillofacial Surgery, Private Practice Suite 3, 241 Adelaide Street, Brisbane, Queensland, Australia
- *Corresponding Author:
- Mark Tuffley
Oral and Maxillofacial Surgery
Private Practice Suite 3
241 Adelaide Street
Brisbane
Queensland
Australia
E-mail: drmarktuffley@denticine.com.au
Received: 16-Feb-2022, Manuscript No. AAOMT-22-54632; Editor assigned: 22-Feb-2022, PreQC No.AAOMT-22-54632(PQ); Reviewed: 20-Apr-2022, QC No.AAOMT-22-54632; Published: 02-May-2022, DOI: 10.35841/aaomt-5.3.111
Citation: Tuffley M. Nutrient-enriched microenvironments: An immunometabolic approach to the management of mronj –A case series. J Oral Med Surg. 2022;5(3):111
Abstract
Objective: To test the clinical effectiveness of adjunctive nutrient augmentation of medicationrelated osteonecrosis of the jaw (MRONJ).
Background: MRONJ represents disordered bone and soft tissue physiology with impaired osseoimmune competence. It often has unacceptable rates of healing particularly in the subgroup of patients suffering osseous malignancies; attempted healing takes place in the context of harsh micro-environmental factors such as hypoxia and nutrient depletions and at times, immune modulating chemotherapy. Immune cell's metabolism is tightly linked to nutrient availability which acts as cues shaping immunological signaling. The resultant immunomodulation is central to adaptive and innate immunity and has profound implications for the healing responses being mediated by myeloid and lymphoid derived cells. We therefore proposed a clinical trial in which we targeted various nutritional sensing pathways via the topical augmentation of MRONJ's nutritional milieu.
Results: 15 consecutive patients were managed over a 7 year period utilizing a comprehensive, nutrient containing hydrogel [CNM] as a wound dressing; this approach was integrated into the overall AAOMS protocol for the management of MRONJ. All patients achieved resolution; the areas of healing remained asymptomatic with intact mucosal coverage for a minimum follow up of 27 months. (Patient 1 in the series improved with a better quality of life before succumbing to her metastatic disease)
Conclusion: The predictable and stable healing responses points to beneficial immuno-modulation of MRONJ's wounds utilising nutritional augmented therapy.
Keywords
MRONJ, Immunometabolism, Chronic wounds, Immunonutrition.
Introduction
As clinicians, we seek more reliable protocols for treating medication-related osteonecrosis of the jaw (MRONJ) [1,2]; it unfortunately is often associated with major repercussions for a patient’s quality of life as well as community health care resources [3,4]. Immune cell function, perturbed in antiresorptive therapy via osteoclastic dysfunction is dependent upon nutrients which act as cues shaping immunological signalling [5]. Unfortunately in chronic wounds such as MRONJ the involved tissues are nutrient scarce and relatively hypoxic. This broadly induces a range of metabolic changes in immune cells as their activity is tightly linked to nutrient availability [6,7]. Stimulating nutrient sensing pathways is a promising therapeutic target as they are integrated into key metabolic regulators such as AKT/mTOR [8- 11]. mTOR adapts cellular metabolism and transcriptional responses in nutritionally favorable micro-environments to promote anabolic cellular activity; for myeloid cells this triggers translational machinery to control development and polarization of effector cells, promoting antigen processing, cytokine production and T cell stimulation [12].
We therefore postulated that the direct application of nutrient enriched, bioactive gels might provoke resolution responses in this chronic wound condition. Hydrogels containing a variety of nutritional stimulants with 0.5% Chlorhexdine gluconate was integrated into the overall management of MRONJ [13]. This trial with nutrient augmented treatment (NAT) was commenced in November 2012 with data cut off in November 2019, having been prompted by disappointing prior outcomes. All together the successful outcome of 15 consecutive patients being treated in this manner continues to prove helpful in the management of these chronic wounds.
Materials and Methods
When considering the pathogenesis of MRONJ in the presence of anti-resorptive therapy, the inhibition of osteoclastic function is central to the dysphysiology of these wounds. Osteoclasts (OC) are osseo-resorptive multinucleated giant cells with important immunological effector functions. They are pleiotropic and regulated similarly to both migratory and resident osteal macrophages (osteomacs) as well as dendritic cells; OCs have recently been identified with different phenotypes and specifically M1, M2 polarizations in osteonecrosis [14,15]. In favorable nutritional microenvironments, osteoclasts together with other myeloid and lymphoid cells orchestrate immune and inflammatory reparative responses including beneficial T cell metabolic activity [5,6,12,16-19]. Downstream this induces a cascade of specialized pro-resolution lipid mediators (SPMs) and other potent cytokines [20-22].
The modulation of the innate and adaptive immune responses is central to wound healing and a return to tissue homeostasis [23-25]. It necessarily includes the recruiting of mesenchymal stem and vascular progenitor cells from the periosteum, bone marrow and circulation which is likely to explain the proresolution cellular changes seen in resolving MRONJ [26]. Most importantly, therapeutically targeting nutrient sensing pathways would seem not to impede the broad range of M1-M2 hybridizations nor unbalance any of the wound’s physiological checks and balances. In testing our hypothesis we formulated a complex mixture of small molecule metabolites with known nutrient sensing triggering capability; these include glucose, glutamine, leucine, arginine and glycine combined with a range a water soluble coenzymes, cofactors and other micronutrients in a hyaluronic acid, glycerol gel. This is combined with 0.5% chlorhexidine gluconate in a pH 7.4 millieu having acid and alkaline buffering properties via balanced amino acid contributions.
The MRONJ wounds were managed according to the AAOMS protocol with areas of inflammation and exposure being adjunctively treated with daily application of NAT. Surgery for non-accessible MRONJ is undertaken after a period of antibiotic stabilization (Generally Doxycycline daily as tolerated]. The hydrogel is also liberally applied per-operatively to the exposed bleeding base of the wound following wide debridement. Surface application of NAT to the healing wound is continued daily until resolution is complete.
Results and Observations
All patients in this series demonstrated resolution with a minimum follow up of 27 months, apart from Case 1 who experienced partial resolution (one of the three areas of exposure spontaneously sequestrated) and a better quality of life before succumbing to her metastatic disease. Eight patients were being treated for malignancy, the remainder osteoporosis. Nine cases (see table 1) with discrete areas of largely accessible, exposed necrotic bone were treated with daily NAT together with adjunctive antibiotics if painful; spontaneous sequestration occurred in 7 patients (2-13 months, average 6.8) leaving a healthy granulation base. The other two proceeded to open surgery having more extensive, inaccessible submucosal necrosis. NAT was continued until complete coverage was evident. All areas have remained stable even in cases with continuation chemotherapy. Antiresorptive therapy had usually been ceased by the Oncologist prior to referral for those patients being managed for osseous malignancy. None of the patients in this series had suffered crush vertebral or other fractures prior to or during the antiresoptive therapy abstinence. Reinstatement of this therapy following healing was encouraged for all particularly for those actively treated chemo-therapeutically.
| No: | Gender-age | Medical Diagnosis and anti-osteoclastic therapy | Initiating Event | Stage | Treatment and Comments |
|---|---|---|---|---|---|
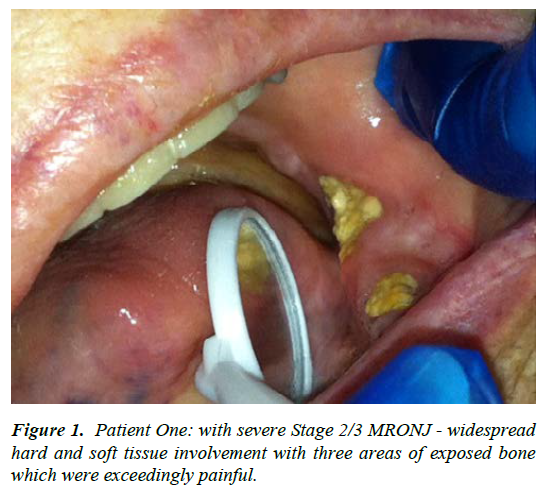
| 1 | F-48 | Metastatic breast and colon carcinoma; IV bisphosphonates for greater than 3 years [estimated] - ceased prior to presentation, November 2012 | Uncertain, although the patient had been edentulous for many years [? full lower denture trauma] | Stage 2/3 | The patient was severely cachectic upon presentation November 2012, with excruciating oral pain - surgery was not considered an option. Utilising CNM cream topically with two, 3 week courses of Doxycycline in addition to her existing analgesic regime gave satisfactory pain control [such that she went camping with her family over Christmas] - see figure 1. Patient passed away mid 2013 with one of the three areas having spontaneously sequestrated - a healing granulation tissue base was noted at 9 months with continued CNM application |
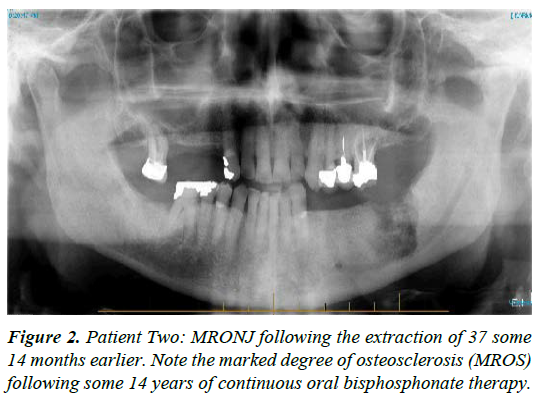
| 2 | M-89 | Osteoporosis treated with 70mg Alendronate, weekly for 14 years, ceased mid 2013. | Extraction of 37 early 2013 by GDP with subsequent non healing of the socket; presentation some 14 months later | Stage 3 | Area progressively worsened with orthodox treatment [see figure 2, mid 2014; note the marked medication related osteosclerosis, MROS] together with moderate ongoing pain. CNM cream helped stabilise both pain and tissue quality with surgical sequestrectomy undertaken under LA, 6 months following presentation; healing 3 to 4 months later with continued CNM cream application; treatment time 10 months |
| 3 | F-69 | Advanced metastatic breast cancer; Denosumab commenced February 2016 and ceased in March [2 doses] | Spontaneous exposure of mandibular lingual alveolus following administration of Everolimus, a mTOR kinase inhibitor | Stage 2 | The patient was in severe pain upon presentation, June 2016 with difficulty eating - all standard measures had proved ineffective. CNM cream proved highly effective in this case such that antibiotics were declined. Spontaneous sequestrectomy occurred in December 2016 with full resolution. Denosumab has been subsequently recommenced; treatment time 5 months |
| 4 | F-62 | Osteoporosis treated with 2 years; 6 monthly Denosumab injections | Spontaneous exposure of lingual mandibular alveolus, 2 weeks following Denosumab administration, February 2017 | Stage 2 | Patient was in considerable pain requiring administration of CNM cream upon presentation together with periodic antibiotic courses; most of the sequestrum was lost in January 2018 with a small spicule lost in March 2018; treatment time 13 months |
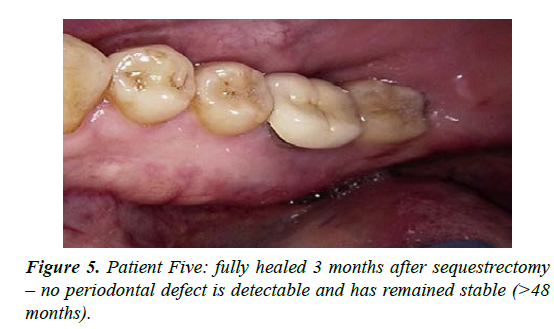
| 5 | M-51 | Early onset osteoporosis treated for 20 years with oral bisphosphonate then switched to Denosumab 6 monthly for 2 years | Spontaneous exposure of mandibular lingual alveolus - presented July 2018 | Stage 2 | Painful on presentation; good pain control with CNM cream together with episodic antibiotics. Area sequestrated in February 2019 with full resolution. Commenced low dose aspirin in an effort to aid healing. Moderate MROS noted radiographically; treatment time 10 months |
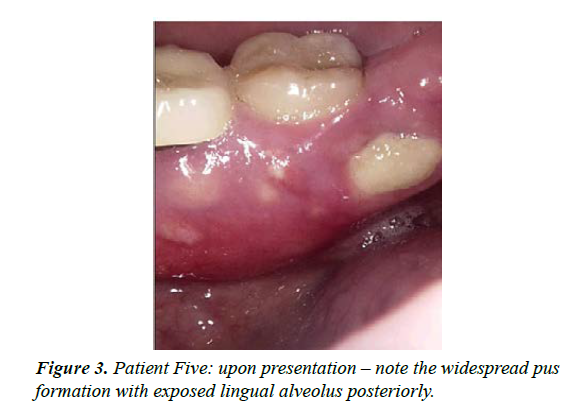
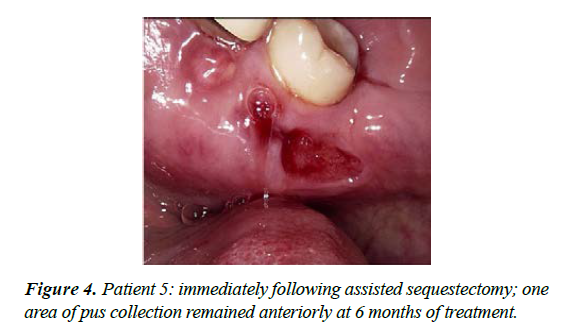
| 6 | M-69 | Multiple myeloma treated with IV Zolendronic acid for more than 2 years; failed to inform GDP who extracted 36 | ‘Dry socket' with non-resolution and exposure of alveolar bone, 4 months duration [see figure 3] | Stage 2 | Moderately painful upon presentation; maintained CNM cream applications and Doxycycline 100mg daily - OH not always optimal with periods of neutropaenia. Dexamethasone, Lanalidomide and low dose aspirin; treatment time 9 months |
| 7 | M-71 | Metastatic prostate cancer -treated with monthly Denosumab injections, commencing 2015 with the last November 2017 combined with two anti-androgenic medications | Referred June 2017 for a symptomatic horizontally impacted 38; 6months following this surgery, Garres osteomyelitis type symptoms with buccal expansion were present | Stage 0 | Open exploration was undertaken in March 2018 which demonstrated reactive new bone with inflammation and fibroblastic proliferation and no evidence of osteoclastic activity -subsequently has remained stable; treatment time 9 months |
| 8 | F-62 | Multiple myeloma [active] with persistent nuetropaenia and marginally low lymphocytes. Monthly IV Zometa commenced 2010 and ceased in March 2013 | 37 surgically removed in August 2017 with a relatively slow healing response - exposed painful lingual alveolar ridge, March 2018 | Stage 2 | Treated with CNM cream and periodic Amoxycillin - good pain control. Patient continued with Lanalidomide and Dexamethasone with symptoms of hypoadrenalism and unstable diabetic control; monthly Denosumab. White cell count presently 1.7x10/9 /L;. treatment time 5 months. |
| 9 | M-84 | Multiple myeloma in remission; Palmidronate IV for 20 years, ceased in September 2018 | Presented May 2018 -15 vertically fractured with an endo-perio lesion ; non painful - exposed bone distally for more than 6 months [see figure 6] | Stage 3 | Operation January 2019 - large amount of non-vital bone [2x2 cm plus] with a sizable antral communication- microscopy showed necrotic bone, granulation tissue and filamentous organisms. Healing proceeded well with a small sequestrum removed in March 2019 - moderate to dense MROS noted; treatment time 10 months |
| 10 | M -67 | Multiple myeloma [active] being treated with chemotherapeutics; 3 monthly Zolendronic acid for 3.5 years with last dose March 2018 | Surgical removal of 25 in March 2018 with confirmed PA inflammation; a partial denture was fitted subsequently by the GDP which resulted in ulceration and exposure of bone distal to 24 | Stage 1 | Managed by not wearing PU denture, relieving the pressure necrosis plus daily CNM cream [no Zolendronic acid was administered in June]; area resolved in 10 weeks without a sequestrum forming |
| 11 | M-72 | Metastatic prostate cancer being treated with anti-androgenic medications, seven rounds of specific chemotherapy, prednisolone and 6 weekly Denosumab commenced March 2018 and ceased in October | Extraction of 37 in December 2018 following jaw pain in this region; slow healing with the area demonstrating continuing low level inflammation [no fistula evident] with subsequent breakdown of lingual/mylohyoid ridge | Stage 2 | Area was managed with antiseptic mouthwashes, CNM cream tds and periodic antibiotics with generally good pain control. Surgical debridement was undertaken after 6 months with the removal of a non-functional 38, attached necrotic bone down to the mylohyoid ridge as well as a chronically inflamed granuloma in the 37 area; fully healed 4 weeks PO - 7 months overall treatment time |
| 12 | F-65 | Severe osteoporosis having been treated previously with Risedronate for 3 years then switched to Denosumab for 2 years, the last dose given one month before presentation. Patient is also treated for SLE with immune therapy and the secondary effects of renal impairment | Area of exposed bone 37 region moderately painful, having been present for some months; predisposing factor likely to be an ill-fitting PL, opposed by a lone standing 27 | Stage 1 | Treated with mouthwashes and CNM cream, together with Doxycycline although subsequently not well tolerated, switched to low dose Penicillin PRN for additional pain control. Spontaneous sequestration at 6 months. Incidentally the patient's Endocrinologist substituted Teriparatide, pulsed dosage PTH analogue with pleasing effect and is now being considered for alterative anti-resorptive medication - Note: no mention of anti-resorptive medication on medical history -approx. 6 months treatment time. |
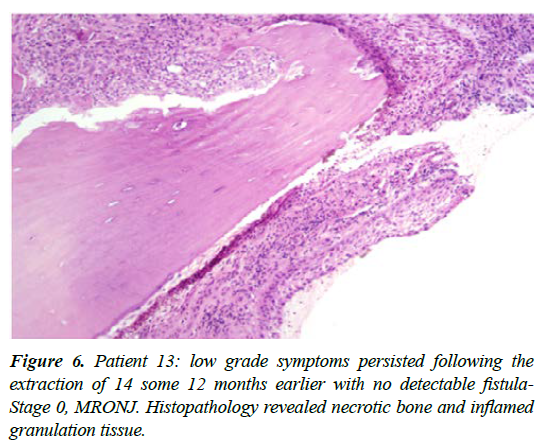
| 13 | M 81 | Metastatic prostate cancer -treated with Zoledronic acid up to 2016 then monthly Denosumab injections; ceased mid 2018 | Extraction of 14, mid 2018 with the area remaining mildly symptomatic, without a discernable fistula | Stage -0 | Wide excision of the 14 site with localised ostectomy in August 2019; histopathology revealed necrotic bone with no evidence of osteoclastic activity [How ships lacunae] together with intensely inflamed granulation tissue. Necrotic bone was extensively eroded by the inflammatory reaction; above treatment resulted in prompt resolution, 4 weeks PO. |
| 14 | F 86 | Osteoporosis: treated with Zolendronic acid following a hip replacement in 2014 followed by Denosumab in 2016 ceased in January 2019 | Extraction of a grossly periodontally infected 13, June 2019; area did not heal with continuing discharge although not painful | Stege-1 | Wide excision in August 2019 with localised ostectomy; demonstrated necrotic bone and granulation tissue with actinomyces species [negative staining for fungus] with acute inflammation; fully healed, treatment time 3 months |
| 15 | F 69 | Osteoporosis: treated with 6 monthly Denosumab for 3 years, last dose, April 2019; variable use of Prednisone for severe eczema | Spontaneous exposure of the 37 lingual alveolar ridge in May, 2019 | Stage-1 | Application of CNM cream with relief of previously variable pain; spontaneous sequestration with full epithelialisation upon subsequent review - treatment time two months |
Table 1. MRONJ Patient Profile - 2012 to 2019
Discussion
The above case series points to outcomes which compare favorably with the many reports of variable but often disappointing rates of MRONJ resolution, a condition described by On S-W et al [27] in 2021 as still being ‘refractory’. Ristow et al [28] reported that early staged lesions managed non surgically generally did not resolve but tended towards progression, a view supported by El-Rabbany et al [3]. In a 2019 Japanese case and literature review [29], resolution of the disease was achieved in 137 out of 275 MRONJ patients (49.8%). One-year cumulative curative rates were 39.8% in stage 1 patients, 26.3% in stage 2, and 19.0% in stage 3. Schiodt M et al, 2017 [30] reported an international multicenter study of patients with MRONJ in the presence of advanced cancer, just over a third of patients remaining at the conclusion of the study were considered to have the osteonecrosis resolved.
We note with interest that two cases treated beyond the data cut off with continuing chemotherapy for progressive metastatic disease developed second, contralateral areas of MRONJ of the mandible, one spontaneous the other via trauma from an ill-fitting denture. This apparent resistance to further breakdown of the original area of osteonecrosis may point to a degree of adaptive immunity being induced with cellular repopulation via the mesenchymal cell, macrophage immunometabolic axis (Figures 1-6).
Conclusion
Although this case series is relatively small, it does support the adjunctive use of NAT in the management of these chronic wounds. The predictability of the observed pro-resolution wound responses via both non-surgical (spontaneous sequestrectomy) and open surgical interventions is most welcome. The resulting stability of wound healing points to effective physiological and immunologic processes beneficially influencing outcomes. Overall, the NAT approach integrated into the management of MRONJ suggests the immuno-metabolism of these wounds can be positively supported towards resolution.
Acknowledgements
Professor Frank N T Monsour’s continued advice, encouragement and proof reading of manuscripts is received with deep gratitude. Collaboration with and therapeutic trials undertaken by Prof K Khosrotehrani, Dermatologist, Clinical Researcher, NHMRC Fellow and the Singaporean Defence Scientific Organisation are most gratefully acknowledged.
References
- Nicolatou-Galitis O, Schiodt M, Mendis RA, et al. Medication-related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis and treatment. OralSurg, OralMed, OrlaPath, OralRadiol. 2019;127:117-35.
- Rosella D, Papi P, Giardino R, et al. Medication-related osteonecrosis of the jaw: Clinical and practical guidelines. J Int Soc Prev Community Dent. 2016;6(2):97-104.
- El-Rabbany M, Lam DK, Shah PS, et al. Surgical management of medication-related osteonecrosis of the jaw is associated with improved disease resolution: a retrospective study. J Oral Maxillofac Surg. 2019;77(9):1816-22.
- McGowan K, Ivanovski S, Acton C. Osteonecrosis of the jaws: a 14 year retrospective survey of hospital admissions. Aus Dent J. 2018;63(2):202-7.
- Walls J, Sinclair L, Finlay DK. Nutrient sensing, signal transduction and immune responses. Semin Immunol. 2016;28(5):396-07.
- Kedia-Mehta N, Findlay DK. Competition for nutrients and its role in controlling immune responses. Nat Commun. 2019;10(1):1-8.
- Lu L, Yao Z. Inflammation and wound healing: the role of macrophages. Expert Rev Mol Med. 2011;13:23.
- Jewell JL, Russell RG, Guan K-L. Amino acid signaling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14(4):133-9.
- Huang J, Manning B. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412(2):179-190.
- Dunlop EA, Tee AR. mTOR and autophagy: a dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol. 2014;36:121-9.
- Weichhart T, Hengstschlager M, Linke M. Regulation of innate immune function by mTOR. Nat Rev Immunol. 2015;15(10):599-614.
- Kang S, Kumanogoh A. The spectrum of macrophage activation by immunometabolism. Int Immunol. 2020;32(7):467-73.
- Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw – 2014 update. J Oral Maxillofac Surg. 2014;72(10):1938-56.
- Wehrhan F, Moibius P, Amann K, et al. Macrophage and osteoclast polarization in bisphosphonate associated necrosis and osteoradionecrosis. J Cranio-MaxfacSurg. 2017;45(6):944-53.
- Madel MB, Ibáñez L, Wakkach A, et al. Immune function and diversity of osteoclasts in normal and pathological conditions. Front Immunol. 2019;10:1408.
- Johnson MO, Sisk PJ, Conteras DC, et al. Nutrients to feed a T cell army. J Smim. 2016;28(5):505-13.
- Wei J, Raynor J, Nguyen T-LM, et al. Nutrient and metabolic sensing in T cell responses. Front Immunol. 2017;8:247.
- Covarrubias AJ. The role of nutrient sensing in macrophage polarization. (Doctoral dissertation).
- Kelly B, O'neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25(7):771-84.
- Serhan CN. Pro-resolving mediators are lead for resolution physiology. Nature. 2014;510(7503):92-101.
- Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128(7):2657-69.
- Duffney PF, Falsetta ML, Rackow AR, et al. Key roles for lipid mediators in the adaptive immune response. J Clin Invest. 2018;128(7):2724-48.
- Lu L, Yao Z, Goodman SB. Inflammation and wound healing: the role of macrophages. J OMS. 2019;77:1816-22.
- Wculek SK, Dunphy G, Heras-Murillo I, et al. Metabolism of tissue macrophages in homeostasis and pathology. Cell Mol Immunol. 2021;1-25.
- Maruyama M, Rhee C, Utsunomiya T, et al. Modulation of the inflammatory response and bone healing. Front Endocrino. 2020;11:386.
- Pajarinen J, Lin T, Gibon E, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80-9.
- On SW, Cho SW, Byun SH, et al. Various therapeutic methods for the treatment of medication-related osteonecrosis of the jaw (MRONJ) and their limitations: A narrative review on new molecular and cellular therapeutic approaches. Antioxidants. 2021;10(5):680.
- Ristow O, Otto S, Troelzsch M, et al. Treatment perspectives for medication-related osteonecrosis of the jaw (MRONJ). J Cranio-Maxfacial Surg. 2015;43(2): 290-3.
- Yamada Si, Kurita H, Kondo E, et al. Treatment outcomes and prognostic factors of medication-related osteonecrosis of the jaw: a case- and literature-based review. Clin Oral Invest. 2019;23(8):3203–11.
- Schiodt M, Vadhan-Raj S, Chambers MS, et al. A multicenter case registry study on medication-related osteonecrosis of the jaw in patients with advanced cancer. Support Care Cancer. 2017;26(6):1905-15.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref