Case Report - Current Pediatric Research (2017) Volume 21, Issue 2
Nosocomial aural myiasis in a sixteen-hour old-Indonesian newborn
Abed Ricky Hernando1, Ekawaty L Haksari1, Alifah Anggraini1, Samad1, Setya Wandita1, Tunjung Wibowo1, Tridjoko Hadianto2, Siswanto Sastrowiyoto31Neonatology Division, Department of Child Health, Faculty of Medicine, Universitas Gadjah Mada, Sardjito General Hospital, Yogyakarta, Indonesia.
2Department of Parasitology, Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia.
3Department of Otorhinolaryngology, Faculty of Medicine, Universitas Gadjah Mada, Sardjito General Hospital, Yogyakarta, Indonesia.
- *Corresponding Author:
- Abed Ricky Hernando
Neonatology Division, Department of Child Health
Faculty of Medicine, Universitas Gadjah Mada
Sardjito General Hospital, Yogyakarta, Indonesia.
Tel: 6281214142900
E-mail: abed_ricky17@yahoo.com
Accepted date: February 22, 2017
Abstract
We reported a case of a two day old newborn who was referred to our hospital due to larvae findings on her left ear. This full-term baby was born to a multigravida mother by spontaneous delivery. She was born active with spontaneous crying. At her first 16h of life, a batch of larvae was found on her left ear. Ear irigation with normal saline resulted in the evacuation 15 larvae from the ear canal. The newborn was jaundiced without any clinical signs of sepsis. Another big larvae crawled out of the ear canal 6 h after being instilled by ear drop. The larvae was investigated in the parasitology laboratory and identified to be Sarcophaga species. Diagnosis of auricular myiasis was established. Routine aural toilet and topical antibiotic was given to the baby. Otoendoscopic examination revealed no other larvae left in the ear canal. The newborn was discharged after 48 h of hospitalization and her condition improved significantly. Neonatal myiasis was considered to be a rare case and usually happened in the environment with poor hygiene.
Keywords
Aural myiasis, Nosocomial, Newborn.
Introduction
Myiasis is an infestation of the dipterous larvae on living vertebrae (humans and/or animals). Worldwide distribution of myiasis has been known. It is considered to be the fourth most common travelling disease. It is also listed in the five most common dermatologic manifestations, accounted for 7.3% to 11% of all cases [1]. Myiasis mostly happened in poor socioeconomic region of tropical and subtropical countries. Aural myiasis is one of the type of cavity myiasis. Myiasis mostly caused by Calliphoridae, Rhinoestris spp., Gasterophilus spp., Hypoderma spp., Chrysina spp. and Sacropagidae spp. In a lesser degree, certain species which also caused myiasis were Anisopodidae, Prophilidae, Stratiomyidae and Syrphidae [2]. An increase of 8% in the mortality rate was reported if aural myiasis was combined with nasal myiasis [1]. Nosocomial myasis is a manifestation of myasis in a hospital setting. Neonatal myiasis is a rare case and considered to be a repugnant disease for the patients. Six percents of wound myiasis occurred in the hospital or nursing home [3]. It is reported that 37.9% of 254 myiasis cases in India occurred in children. Some identified risk factor of myiasis was poor hygiene, low economic status, deterioration of level of consciousness, hypoesthesia, paralysis, and immobility condition [4,5]. Two of the most common infection sites of neonatal myiasis were umbilical cord and foreskin. Some studies reported other infection sites such as open wound, nasal, nasopharyngeal, ocular, auricular, urogenital area, and in nasogastric tube [5-13].
Case Report
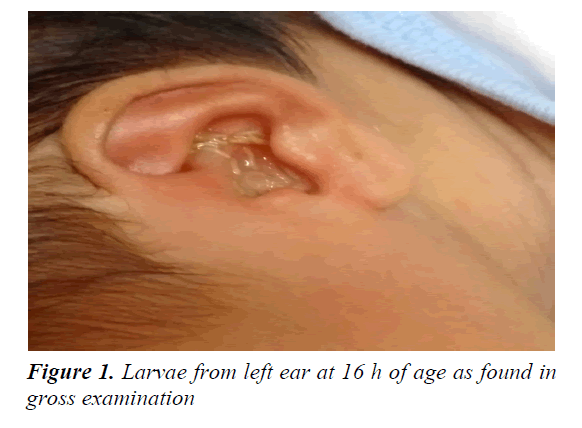
A two day old newborn was referred from a private hospital outside the city to our tertiary teaching hospital, due to larvae found on her left ear. The larvae was first observed crawling out from her left ear at the 16 h after birth. This full term baby was born to a multigravida mother by spontaneous delivery, with 1 min Apgar score of 7 and 5 min of 9. On admission, the baby weighed 3300 g with 39+5 weeks of gestational age (Dubowitz score), her body temperature was 36.6°C, respiratory rate was 56 breaths/ min and heart rate was 132 beats/min. She was active, cried spontaneously, not lethargic and her fontanelle was soft. Larvae with serous debris was seen coming out of her left ear (Figure 1). Her left ear was then drainaged with normal saline solution. Fifteen larvae were then evacuated from her left ear. Ear examination revealed laceration and blood clot in the left auditory canal. An intact tympanic membrane was visualized during the examination.
Laboratory findings of this newborn showed a white cell count of 24.440/mm3 consisting of 63.7% neutrophil and 21.6% lymphocyte; also total bilirubin of 10.74 mg/dl. The neonate developed jaundice but there was not any clinical sign of sepsis observed. However, phototherapy was not given to the baby. Another big larvae crawled out of the same ear canal 6 h later, after the affected ear was being instilled by ear drop. The sample of the larvae was sent to the parasitology laboratory for further examination. The result was the identification of 24 to 48 h old Sarcophaga sp., in the stage of 2nd instar (Figure 2). The patient’s biological mother was consulted to the Obstetry and Gynecology Department to exclude intrapartum infestation of the larvae. The examination showed that her vulva and vagina were in the clear condition.
Eardrop containing Chloramphenicol, Polymyxin B sulphate, Benzocaine, Nipagin, was given every 8 h in the left ear; and aural toilet was performed every 24 h. Systemic antibiotic was not administered because the condition of the patient did not fulfill the clinical sign criteria of sepsis. Tetanus toxoid was not given to this newborn. The baby still looked active, breastfed well, and did not develop any fever. After 48 h of hospitalization, the patient was discharged with no clinical sign of sepsis and clear left ear. On the following 24 h of discharged period, the newborn visited neonatal and otorhinolaryngology outpatient clinic and underwent follow-up otoendoscopic examination. The examination revealed that there was not any infestation of larvae and intact tympanic membrane was visualized. The newborn was active, showed no clinical signs of infection, and reported that had no problem in breastfeeding. No additional therapy was given on this follow-up examination. MRI examination was not carried out due to the intact tympanic membrane. The newborn was asked to visit otorhinolaryngology outpatient clinic 1 week later and had good condition.
Discussion
Myiasis is defined as an infestation and invasion of living vertebrates (human and/or animal) tissue by dipterous larvae. Any cutaneous tissue, body cavity, or body organ could be attacked by these larvae [1,6,7]. Myiasis in neonates commonly occurred at umbilical cord and foreskin [7,8]. Aural myiasis occurred infrequently and mostly found affecting children or adult with mental retardation [6].
Only a few incidences of neonatal aural myiasis were reported [5,9,10]. Bapat reported a case of aural myiasis caused by Calliphoridae with secondary bacterial infection in an orphan fullterm neonate, who was abandoned in a dubstin in Poona, India [9]. Kamble also reported a case of aural myiasis in a 17 day old healthy neonate without any predisposing ear complaint [10]. The maggots were identified as Calliphoridae sp. In Cetinkaya‘s report, a 12 day old female infant suffered from aural and ocural myiasis and jaundice [5].
The most important risk factors for myiasis were poor hygiene and low socioeconomic status. Higher incidence of myiasis was discovered in rural zones of tropical and subtropical areas [11]. Deterioration of level of consciousness, hypoesthesia, paralysis and immobility also increase the risk of myiasis [5]. Some predisposing factors for myiasis were weakness or debilitated condition, presence of open wound or necrotized tissue, inadequate hygiene or nursing care, absence of screen on windows and summer season (warm climate) [12]. Comatose and handicapped patients were also in higher risk of myiasis. Reported cases of myiasis were found on open wound, nasal, nasopharyngeal, ocular, auricular, urogenital area and in the nasogastric tube [1].
In this case, the newborn was transported to a transition room after receiving a routine neonatal care. The newborn stayed there and was observed for 3-4 h before transported to the mother’s room for rooming in. Larvae were first found when the newborn 16 h old. A larvae sample was brought to the parasitology laboratory when the newborn was 40 h old. The result was the identification of 24 to 48 h old Sarcophaga sp., in the stage of 2nd instar. We suggested that infestation of the parasites began when the baby was placed in the transition room. This hypothesis was in line with the confirmation of the larvae’s age.
Patients with aural myiasis commonly presented with larvae on external ear canal, aural malodor, purulent or hemorrhagic aural discharge, ear pain and itching [6]. Adult or older children may also complained of tinnitus and vertigo. The infestation was usually unilateral as seen in this patient, but it could be bilateral. On otoscopic examination, the tympanic membrane may be intact or perforated. The injury of the auditory meatus may lead to deafness, meningitis or even death [6]. Myiasis of ears and nose are dangerous because of the capability of the larva to penetrate into the brain and the tissue damage, due to inflammatory reaction and secondary bacterial infection. Case fatality rate of this condition is 8% [5].
Treatment with turpentine oil on the affected ear followed by manual removal of the larvae along with administration of systemic and topical antibiotic is the treatment chose in most reports [6,13-20]. Irrigation of the ear with saline, 70% ethanol, 10% chloroform, normal saline, oil drops, urea, dextrose, creatine, topical ivermectin or iodine saline has been used to help remove the maggots [1]. We received this patient in the emergency room, which had no turpentine oil available. According to Francesconi, normal saline also could be used in this setting. Thus, our otorhynolaryngologist decided to irrigate the ear canal with normal saline immediately.
Tetanus Toxoid injection was not given to the newborn, as the immunization status of the mother was TT4. The purpose of giving that vaccine for women of childbearing age and to pregnant women is to protect them from tetanus. Besides that, the same protective effect is also directed to their newborns. The expected protection duration of women with TT4 status is at least 10 years [20]. According to the maternal vaccination status and previous clean delivery which she had, we decided to not giving tetanus toxoid to this newborn.
The newborn did not undergo any phototherapy. According to the clinical practice guideline from American Academic of Pediatric on Hyperbilirubinemia, the phototherapy treatment limit for this age was 13.0 mg/dl [18].
We did not find any clinical septic signs shown from this newborn (observed from her general condition, central nervous, cardiovascular, respiratory, and hematologic system). Jaundice was the only abnormal condition we found since the baby was admitted to the emergency department. This jaundice suggested a physiologic hyperbilirubinemia, so we decided to do the watchful waiting to the baby for her condition. According to the standard procedure of our hospital, the systemic antibiotic was not indicated.
MRI examination was not carried out due to the intact tympanic membrane. MRI procedure has been used in a number of cases such as cerebral myiasis; breast myiasis; also facial, orbital, and furuncular myiasis [19]. Since the intact tympanic membrane was found, intracerebral infection was not indicated on this reported case. Besides, the high cost of this imaging procedure burdened the patients financially.
Nosocomial myasis is an infestation of larvae that occurred in the hospital setting. Although the case was rare in developed countries, it may be underreported in developing and poor countries [1].
The causative agents of nosocomial myiasis were Lucilia sericata, Megaselia scalaris, Sarcophaga spp., C. hominivorax, Cochliomyia macellaria and M. domestica. Flyblows of Sarcophagae were known to cause necrotic myiasis [15]. Most of the identified agents were Sarcophagidae family. Among the Sarcophagidae species, Wohlfahrtia magnifica is frequently seen as a causative agent of various types of myiasis in the Mediterranean Basin, South Russia, Turkey, Israel and the Middle and Far East countries [15]. Nosocomial aural infestation of Sarcophagidae in neonate had also been reported in Malaysia [13].
Although Sarcophagidae are commonly larviparous, depositing larvae directly onto a breeding medium, some species occasionally lay eggs. In the absence of the certain features attracting the species to induce myiasis (exposed wounds, necrosis, ulcerations, etc.), a female Sarcophaga laid larvae in an anatomical cavity that doesn’t have a continuous water flow. Then the larva begins eroding epidermis, causing a fissure. This larva typically feed on carrion. Some studies reviewed the speed of growth of this orde (Sarcophaga dux). In Malaysia the average developmental period of the second instar, third instar, postfeeding and pupa took 19, 40.5, 73 and 91 h, respectively, based on the fluctuative temperature [16]. An important gross taxonomic feature in this family is the nature of the posterior spiracle located within a depression/pit at the posterior end of the maggot. The other distinct features of Sarcophagidae are the possession of thin incomplete peritreme with respiratory slits directed away from the opening, and the cephalopharyngeal armature with the split of dorsal cornu [16]. In identifying the larval stages, special attention was given to the ultrastructure of the anterior and posterior spiracles. In the first instar larvae, the ventral cornua were shorter than the dorsal cornua. In the second instar, the length of the ventral cornua was much shorter than that of the dorsal cornua. The dental sclerite in the first, second and third instar larvae gradually reduce in size. In third instar larva, the distinct feature were the dorsal spines between the first and second thoracic segments [17]. Sarcophagidae commonly causes wound myiasis and furuncular myiasis although ocular, oral, and otomyiasis had also been reported [1,15].
Nosocomial myiasis may alert one to the presence of another infestations, as exemplified by the case of a hospital with a mouse infestation (mouse carcasses could have attracted flies) [1]. Prevention of nosocomial myiasis includes taking control of the fly population, maintaining general cleanliness, and reducing the odors of decomposition. Wound cleaning and protecting, as well as umbilical cord care should also be taken into serious consideration. Presumed environmental access site should be closed and insecticide spray should be augmented. Any open wound should be desinfected and covered. Nets for windows, UV-A traps and other ventilation ducts are simple tools that could help in preventing nosocomial myiasis. Hospital infection control unit should provide mosquito nets around the high risk patients’ bed [12]. Storage and food preparation station should also be reviewed [1].
Summary
The cause of nosocomial aural myiasis in this newborn was Sarcophagidae sp. Early recognition of the larvae and the early management of the case had an important role in preventing any further complications. Evaluation of the hospital sanitation, also the alertness of the medical personnel and officers about environmental hygiene is necessary to prevent the larvae infestation from happening, especially in certain areas such as perinatal ward and other intensive care unit areas.
Acknowledgement
To the parents of our patient, for the coordination during the whole examination process of their newly born daughter. To the Department of Parasitology Faculty of Medicine, Universitas Gadjah Mada. Thank you also to Shianita MD and all doctors and nurses in neonatology ward for their contribution.
References
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev 2012; 25: 79-105.
- Ramana KV. Human myiasis. Medical Microbiology and Diagnosis 2012; 1: 2-3.
- Sherman RA. Wound myiasis in urban and suburban United States. Arch Intern Med 2000; 160: 2004-2014.
- Singh I, Garhwala G, Yadav SP, et al. Myiasis in children: the Indian perspective. Int J Pediatr Otorhinolaryngol 1993; 25: 127-131.
- Ãetinkaya M, Ãzkan H, Köksal N, et al. Neonatal myiasis: A case report. Turk J Pediatr 2008; 50: 581-584.
- Yuca K, Caksen H, Sakin YF, et al. Aural myiasis in children and literature review. Tohoku J Exp Med 2005; 206: 125-130.
- Clark JM, Weeks WR, Tatton J. Drosophila Myiasis Mimicking Sepsis in a Newborn. West J Med 1982; 136: 443-444.
- Duro EA, Mariluis JC, Mulieri PR. Umbilical myiasis in a human newborn. J Perinatol 2007; 27: 250-251.
- Bapat SS. Neonatal myiasis. Pediatrics 2000; 106: E6.
- Kamble BB, Jain S, Gupta M, Singh P. A rare case of neonatal aural myiasis in a 17 days old neonate. Online J Heal Allied Sci 2015; 14: 12-13.
- Goyal S, Dhyani A, Agarwal K. Neonatal umbilical myiasis: Case report. 2015; 1: 40-41.
- Dutto M, Bertero M. Cutaneous superficial myiasis: Report of a rare nosocomial parasitic disease caused by Sarcophaga spp. (Diptera, Sarcophagidae). Cent Eur J Public Health 2011; 19: 232-234.
- Ahmad NW, Ismail A, Jeffery J, et al. Aural myiasis in a neonate in peninsular Malaysia. Parasites vectors 2009; 2: 63.
- Al Jabr I. Aural myiasis, a rare cause of earache. Case Rep Otolaryngol 2015; 2015: 219-529.
- Mielke U. Review: Nosocomial myasis. J Hosp Infect 1997; 37: 1-5.
- Sukontason KL, Sanit S, Klong-Klaew T, et al. Sarcophaga (Liosarcophaga) dux (diptera: Sarcophagidae): A flesh fly species of medical importance. Biol Res 2014; 47: 1-9.
- Chakraborty A, Ansar W, Ghosh S, et al. The first report of the life cycle of Sarcophaga (L) dux on dead reptilian carcass: Their application as forensic indicators. 2014; 2: 731-739.
- Subcommittee on hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004; 114-297.
- Ofordeme KG, Papa L, Brennan DF. Botfly myiasis: A case report. CJEM 2007; 9: 380-382.
- Lincetto O. Maternal immunization against tetanus. WHO Standards for maternal and neonatal care 2002; 1: 1.