- Current Pediatric Research (2016) Volume 20, Issue 1
Noninvasive Assessment of Liver Fibrosis in Egyptian Children with Chronic Liver Diseases.
- *Corresponding Author:
- Azza Youssef
Assistant Professor, Department of Pediatrics
Faculty of Medicine, Ain Shams University
Cairo 11727, Cairo, Egypt
E-mail: azzayoussef@med.asu.edu.eg
| Date of Acceptance | May 17, 2016 |
Abstract
Aim: to evaluate Liver stiffness measurement LSM and aspartate transaminase to platelet ratio APRI score as non invasive means of fibrosis assessment in children with chronic liver disease.
Methods: Liver biopsy was done for 42 children (20 boys and 22girls with mean age of 11.5 ± 3.9 years) suffering from various chronic liver diseases. The stage of fibrosis was assessed according to METAVIR system. APRI score was calculated for 42 of the children and LSM by transient elastography was performed to each of the children as well as to 18 healthy age and sex matched controls. The correlation between METAVIR fibrosis stage and each of the APRI score and LSM was studied. Cutoff points for differentiation of no or mild fibrosis from significant fibrosis in the group as a whole was calculated for APRI and LSM using ROC curves.
Results: fifteen children had no or mild fibrosis (=F2 METAVIR) (G 2). The mean APRI score of the patients was 0.71 +/- 0.48. It was significantly higher in G2 vs G1 patients (0.71 vs 0.3) and showed significant correlation with METAVIR staging (r= +0.53, P<0.001). At a cut off of 0.58 it had 63%, 73%, 70.4%, 66.7% sensitivity, specificity, PPV and NPV respectively for significant fibrosis. LSM was significantly higher in patients vs controls (38.8 vs 11.1 kPas and P<0.000) and in G2 vs G1 (15.4 vs 6.9 KPas and P<0.000). It showed significant positive correlation with both METAVIR staging and APRI score (r=0.58, 0.69 respectivelyand P<0.001). A cut off of 8.1KPas had a 78%, 73%, 77.8% and 73.3% sensitivity, specificity, PPV and NPV respectively for significant fibrosis.
Conclusion: In children, APRI score and LSM perform reasonably well in fibrosis assessment. Diseases with different etiologies have different cutoff values for significant fibrosis.
Keywords
Liver stiffness measurement, Fibroscan, APRI score, Liver fibrosis, Children
Introduction
Outcome of liver disease in children is largely determined by severity and progression of liver fibrosis. Liver biopsy LB is the accepted standard for evaluating fibrosis but is limited by the need for sedation in children, sampling error, and risks including bleeding [1].
Furthermore, liver biopsy is an invasive procedure, prone to sampling error and has the potential for medical complications. Inter- and intra-observer discrepancies of 10 - 20% have been reported in assessing hepatic fibrosis and may lead to erroneous estimation of cirrhosis [2-4].
Because of the limitations of liver biopsy, there is strong interest in developing less invasive methods to assess liver fibrosis especially in children. Liver stiffness measurement (LSM) by transient elastography( TE) involves transmission of vibrations through the chest wall into the liver using an ultrasound probe with a vibration transducer, the elasticity of the liver is determined from the velocity of the wave [5,6]. The accuracy of TE has been shown to be excellent in a large number of adult studies A few small studies have also been undertaken in children [5-8].
Aspartate transaminase to platelet ratio (APRI) score is simple, readily available and inexpensive [9].
Both are non invasive means of liver fibrosis assessment that have been studied in chronic liver disease adult patients [10,11].
The aim of the present study was to evaluate LSM and APRI score as non invasive means of fibrosis assessment in children with chronic liver disease in general.
Materials and Methods
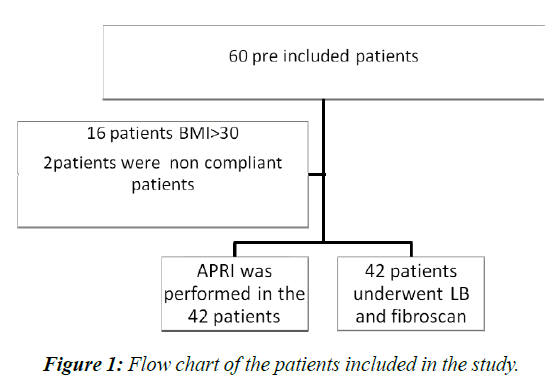
A case control study was done during 14 months period (from Feb 2012 to March 2013), liver biopsy was done for 42 patients (Figure 1). They were 20 boys and 22girls with mean age of 11.5 ± 3.9 years) with chronic liver disease of different etiologies recruited from two tertiary hepatology clinic in Cairo, Egypt (Children ̓s Hospital, Ain Shams University and Yassin Abdel Ghaffar Charity Center for Liver Disease and Research). The stage of fibrosis was assessed according to METAVIR system [12].
APRI score was calculated for 42 of the children and LSM by fibroscan was performed by the same radiologist to each of the children as well as to 18 healthy age and sex matched controls , their mean age of 10.94 ± 4.12 years .
The patients were included according to the following criteria
Inclusion criteria
1. Children with a diagnosed chronic liver disease.
2. Having a liver biopsy done within 6 months from Fibroscan examination.
Exclusion criteria:
1. Presence of ascites.
2. Children with narrow intercostals spaces (defined as thoraco perimetric measurement < 45 cm).
3. Children with acute on top of chronic liver disease.
4. Overweight children (defined as BMI ≥ 30 Kg/m2).
5. Any children with contraindication for liver biopsy.
6. Patients who didn’t give 10 valid measurements on fibroscan examination.
Methods: This study was approved by “the Ethical Committee of Pediatrics Department-Ain Shams University”. This work was carried out in accordance with ‘‘The Code of Ethics of the World Medical Association’’ (Declaration of Helsinki).
For all study participants’, or their legal guardian, provided informed written consent prior to study enrollment.
LSM using Fibroscan: It was performed using ultrasound transient elastography (Fibroscan, Echosens, Paris, France. The device is located in KasrAlaini, Viral Hepatitis Center. The examination was performed by a single operator within 6 months of liver biopsy for all patients and controls. It consists of 5MHz ultrasound transducer probe mounted on the axis of a vibrator. Vibrations of mild amplitude and low frequency are transmitted by the transducer, inducing an elastic shear wave that propagates through the underlying tissues. Velocity of the wave is directly related to tissue stiffness – the stiffer the tissue, the faster the shear wave propagates. The median value of ten successful acquisitions is expressed in kilopascal (KPa) and is kept as representative of liver stiffness measurement and the technique was performed as that described by [13].
APRI score was done by using the following formula:
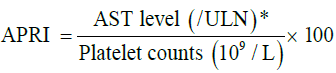
*Results of serum aminotransferase (AST) levels were expressed as ratios of the upper limit of normal (ULN=40 U/L).
The correlation between METAVIR fibrosis stage and each of the APRI score and LSM was studied. Cutoff points for differentiation of no or mild fibrosis from significant fibrosis in the group as a whole was calculated for APRI and LSM using ROC curves.
Statistical analysis: was performed using SPSS 17.0 statistical package. All results were expressed as mean ± SD values for parametric data and median, IQR for non-parametric quantitative data. Student’s t-test was used for means, Mann-Whitney Test for non-parametric quantitative data and Chi-square test for comparing categorical variables. Pearson Correlation and Spearman's rho was used for correlation in parametric and nonparametric data respectively. A P value of < 0.05 was considered significant.
ROC curves (Receiver operator characteristic curves) were constructed to assess cut off value to discriminate between children with (Fibrosis < F2 i.e. no or insignificant fibrosis) and those with (Fibrosis ≥ F2 i.e. significant to sever fibrosis) as assessed by METAVIR scoring system for the whole patients group.
Results
Liver biopsy was done for 42 children (20 boys and 22 girls with mean age of 11.5± 3.9 years suffering from various chronic liver diseases (Table 1).
| Diagnosis | NO. | Percent (%) |
|---|---|---|
| Wilson disease | 16 | 38.1% |
| AIH | 10 | 23.8% |
| GSD | 3 | 7.1% |
| CHF | 3 | 7.1% |
| Cryptogenic cirrhosis | 3 | 7.1% |
| Chronic HCV | 2 | 4.8% |
| Chronic HBV | 1 | 2.4% |
| Drug induced hepatitis | 1 | 2.4% |
| Steatohepatitis | 1 | 2.4% |
| Budd chiari syndrome | 1 | 2.4% |
| PFIC | 1 | 2.4% |
| Total | 42 | 100% |
Table 1: Descriptive data of studied children as regards aetiology of liver disease.
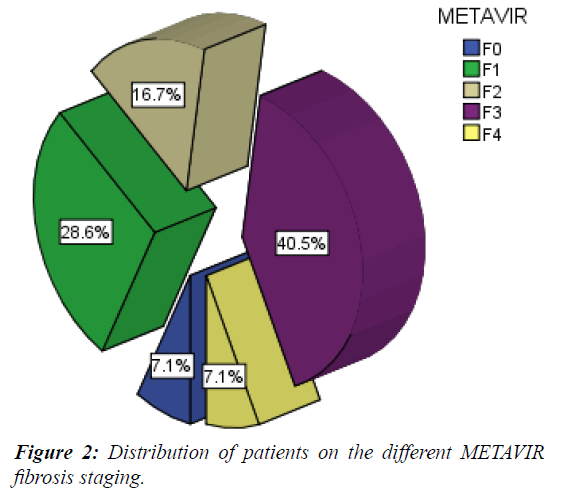
Fifteen children had no or mild fibrosis (<F2 METAVIR score) (G1) while 27 had significant fibrosis (>=F2 METAVIR score) (G 2) (Table 2 and Figure 2).
| Variable | Mean ± SD | Range |
|---|---|---|
| Age (Years) | 11.5 ±Â 3.9 | 3 ? 18 |
| Wt. (Kg) | 38.9 ±Â 13 | 9 ? 60 |
| Ht. (Cm) | 139.5 ± 20.2 | 85 ? 169 |
| BMI(Kg/m2) | 19.2 ±Â 3.03 | 12.5 - 27.6 |
| HB(g/dl) | 11.59 ± 1.38 | 8.2 - 14.1 |
| WBC(x103/μL) | 6.5 ± 2.2 | 3.1 - 12.7 |
| Platelets(x103/μL) | 207.9 ± 90.4 | 54 ? 407 |
| INR | 1.12 ± 0.12 | 1Â -Â 1.48 |
| ALT(U/L) | 40.9±23.11 | 7 ? 82 |
| AST(U/L) | 48.5±23.01 | 14 ? 119 |
| Bilirubin(mg/dl) | 0.71 ± 0.53 | 0.19 - 2.77 |
| GGT(U/L) | 56.87 ± 79.1 | 10 -Â 410 |
| ALP(U/L) | 340.03 ± 240.1 | 34.8 ? 1015 |
| Albumin(g/dl) | 4.2 ± 0.65 | 2.7 - 5.2 |
Table 2: Baseline data of studied patients.
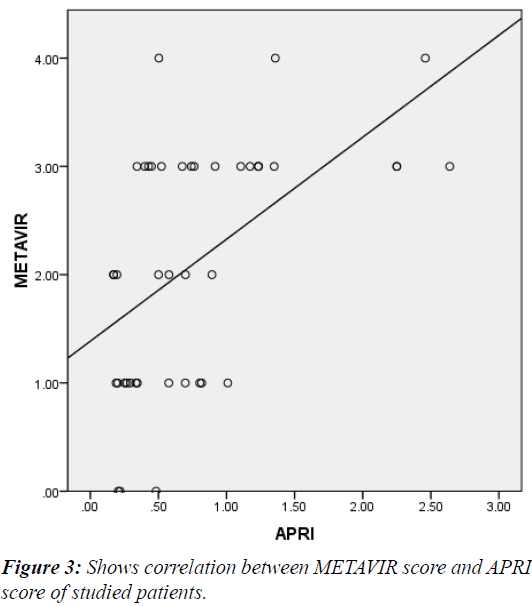
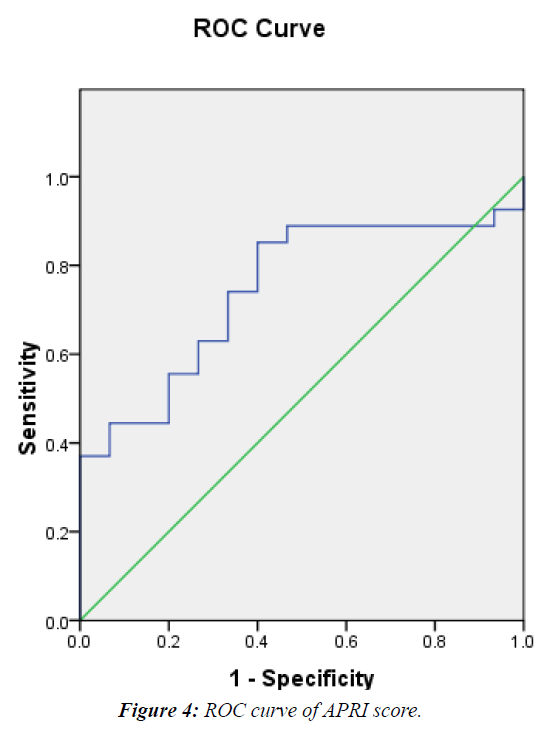
The mean APRI score of the patients was 0.71 +/- 0.48. It was significantly higher in G2 vs. G1 patients (0.71 vs 0.3) P<0.009 (Table 3) and showed significant correlation with METAVIR staging (r= +0.53, P<0.001) (Figure 3). At a cutoff of 0.58 it had 63%, 73%, 70.4%, 66.7% sensitivity, specificity, PPV and NPV respectively for differentiating significant fibrosis (Figure 4).
| Item | Â APRI score | z-test | p-value | Sig. | |
|---|---|---|---|---|---|
| Range | Mean ± sd | ||||
| <F2(n=15) | 0.19 ? 1 | 0.3 ± 0.45 | -2.612 | 0.009 | Sig. |
| ≥F2(n=27) | 0.17 - 2.64 | 0.71 ± 0.96 | |||
Table 3: Comparison between APRI score in both groups of patients (Fibrosis staging by METAVIR scoring).
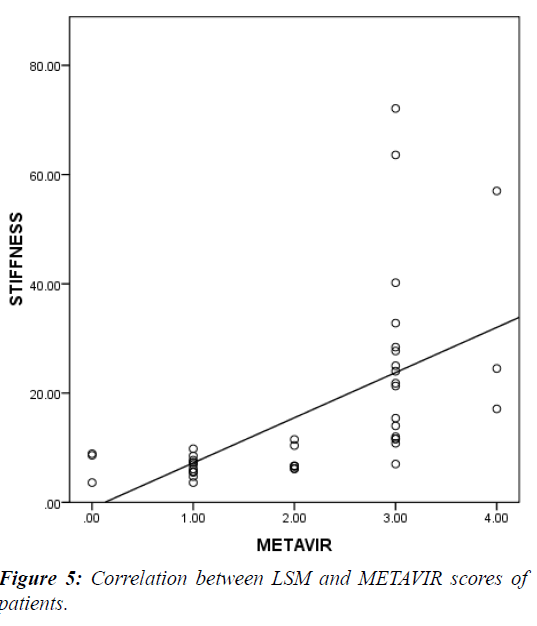
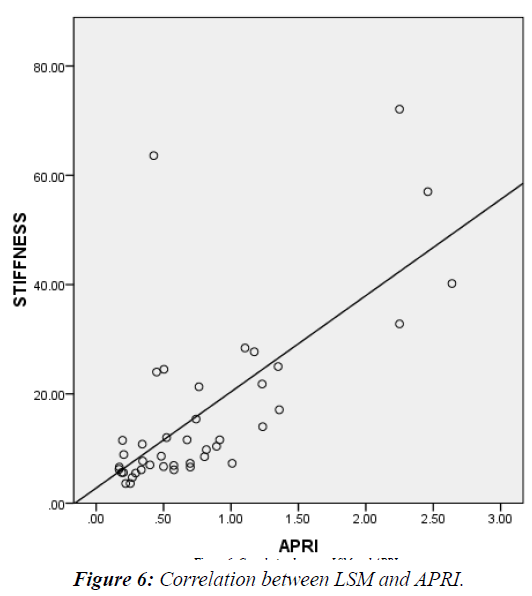
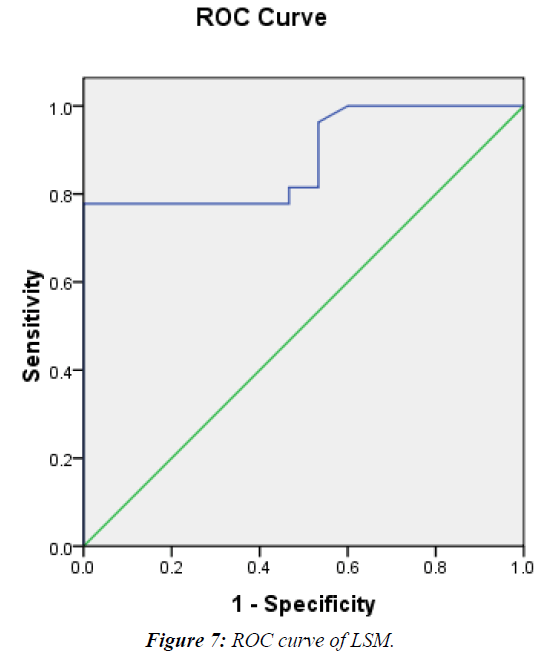
LSM was significantly higher in patients vs. controls (38.8 vs. 11.1 KPa and P<0.000) (Table 4) and in G2 vs. G1 (15.4 vs. 6.9 KPa and P<0.000) (Table 5). It showed significant positive correlation with both METAVIR staging (Figure 5) and APRI score (Figure 6) (r=0.58, 0.69 respectively and P<0.001). A cutoff of 8.1KPa had a 78%, 73%, 77.8% and 73.3% sensitivity, specificity, PPV and NPV respectively for differentiating significant fibrosis (Figure 7).
| Group | LSM (KPAS) Mean rank | Z-test | (p-value) |
|---|---|---|---|
| Diseased (No. = 42) | 38.8 | -5.62 | 0.000 |
| Control (No. = 18) | 11.14 |
Table 4: Comparison between liver stiffness measurement (LSM) of diseased and control groups.
| Item | LSM (KPa) | Z-test | P-value | Sig. | |||
|---|---|---|---|---|---|---|---|
| Min. | Max. | Median | IQR | ||||
| <F2(n=15) | 3.6 | 9.8 | 6.9 | 3 | -4.07 | 0.000 | Sig. |
| ≥F2(n=27) | 6.1 | 72.1 | 15.4 | 17.3 | |||
Table 5: Comparison between LSM by fibroscan in both groups of patients.
Discussion
The present study was designed to evaluate TE performance in children with chronic liver disease. TE was particularly good at distinguishing significant fibrosis from no fibrosis. Although not proposed as an alternative to biopsy for diagnostic purposes, TE could serve as a valuable tool in the long-term follow up of pediatric liver disease. Liver biopsies are rarely repeated following initial diagnostic biopsy in pediatric cohorts and there is a real need for a noninvasive tool that could characterize fibrosis progression or regression.
Our study showed that there is a highly significant positive correlation between fibrosis as assessed in liver biopsy by METAVIR scoring system and liver stiffness measurement by fibroscan (r =0.58 and p-value=0.0001). This is in agreement with a study by which was conducted on 33 children with different chronic liver diseases and which also showed highly significant positive correlation between LSM and METAVIR fibrosis stage (r=0.53 and p value=0.001) [5]. Also found a highly significant positive correlation between LSM and METAVIR fibrosis stage (r = 0.5694 and p-value <0.0001) [14]. Thus according to our results and those of others LSM in children as in adults correlates strongly with histological stage of fibrosis [5].
TE was a good discriminator of fibrosis in the present study, a cut off value of 8.1Kpas could differentiate between significant fibrosis (≥ F2) and absent or insignificant fibrosis (<F2) irrespective of the etiology of chronic liver disease, with AUROC, sensitivity, specificity, PPV and NPV of 0.883, 78%, 73%, 77.8% and 73.3% respectively. This is in agreement with an adult study by on 200 patients with chronic liver diseases who found that a cut-off (7.9kpas) is the cut-off for diagnosis of significant liver fibrosis (≥ F2) with AUROC, sensitivity, specificity, PPV and NPV were 0.86, 72%, 84%, 82% and 70% respectively [7].
In a study done by on 101 children with different chronic liver diseases the cut-off value of 6.9 KPa differentiated between patients with no or insignificant fibrosis (<F2) by METAVIR from those with significant fibrosis (≥ F2) METAVIR with sensitivity 68% and specificity 78% with AUROC was 0.78 [1]. This cut-off value was less than the cut off value in the current study which was 8.1 KPa with higher AUROC and sensitivity and quite similar specificity (0.88, 78%, 73%) respectively.
Also in a study done by on 312 patient more than 18 years old with chronic hepatitis C in Egypt ,they concluded that fibroscan did well, although not as efficient at grade F2and F4 stages than already published studies , with cut off value in stage ≥ F2 of 7.8 KPa with AUROC, sensitivity, specificity, PPV and NPV were 0.71%, 53% , 83% ,PPV 85% and NPV48%. This lower statistical correlation between the FS with grades of fibrosis could be increased using an XL probe in daily practice in Egypt. This should be a particular subject to pay attention for, as 97/312 (31%) of the patients included in this study have a BMI > 30 and this may explain its difference from the present study [14,15].
In the current study there was a highly significant positive correlation between APRI score and stage of fibrosis as assessed by METAVIR scoring system (r=0.53 and p-value =0.000).This was in agreement with a study by on 150 patients with chronic HCV infection in whom liver biopsy was done and assessed by METAVIR scoring system and APRI score was calculated and results showed positive correlation between APRI score and liver fibrosis as assessed by METAVIR (r=0.57 and p-value =0.0001) [16]. Similarly a study by on 33 children with different chronic liver diseases showed positive correlation between APRI score and liver fibrosis stage as assessed by METAVIR (r=0.32 and p-value=0.03) [5].
The current study revealed that there was highly significant negative correlation between LSM by fibroscan and platelet count in the studied patients (r=-0.635 and p-value=0.000).This is in agreement with demonstrated negative correlation between LSM by fibroscan and platelet count (r=-0.44 and p-value=0.001) [5].
In the present study the mean cut-off value of APRI score for patients with fibrosis stage less than F2 (<F2) was (0.45 ± 0.3) while for patients with fibrosis stage equal or more than F2 ( ≥ F2) was (0.96 ± 0.71).This is in agreement with a study by on 120 patients with chronic HCV infection and the mean cut off value of APRI score for fibrosis less than F2 (<F2) was (0.69 ± 0.44) while for patients with fibrosis stage equal or more than F2 ( ≥ F2) was (1.38 ± 0.78) [17].
In the current study ROC curve of APRI score shows that cut off value of (0.58) is the best to differentiate between patients with significant to severe fibrosis (≥ F2) from those with absent or insignificant fibrosis (< F2) with AUROC, sensitivity, specificity, PPV and NPV were 0.746, 63%, 73%, 70.4% and 66.7% respectively. This is in agreement with a study by on 150 patients with chronic HCV infection in whom liver biopsies were done and assessed by METAVIR scoring system, APRI score was calculated and results showed that cut off value of APRI score at (0.52), the best to detect patients with significant to severe fibrosis (≥ F2) with AUROC, sensitivity, specificity, PPV and NPV of 0.766, 70%, 81%, 97% and 24.5% respectively [16].
Also the present study is in agreement with a study by on 192 patients with chronic HCV infection in which 2 cut-off points were chosen to predict the absence (APRI ≤ 0.50) or presence (APRI > 1.50) of significant fibrosis (METAVIR F2-F4) [9]. For the prediction of significant fibrosis in the validation set, the positive predictive value (PPV) and negative predictive value (NPV) of an APRI of 0.50 were 64% and 90%, and the corresponding values for an APRI of 1.50 were 91% and 65%, respectively (41% sensitive and 95% specific).This is similar to a study by on 120 patients with chronic HCV infection and found that also 2 cut off values were chosen to predict absent or insignificant fibrosis (F0-F1) (APRI ≤ 0.5) or presence (APRI>1.5) of significant fibrosis (METAVIR F2- F4) with sensitivity, specificity, PPV, NPV and AUROC of (83%,66%,72%, 78% and 0.82) respectively for APRI ≤ 0.5, but with sensitivity, specificity, PPV, NPV and AUROC were (41%, 95%, 90%, 58% and 0.87) respectively for APRI>1.5 [17].
Also another study was done by on 68 children with chronic liver diseases and they found that APRI score with cutoff value 0.82 is the best to differentiate between children with no fibrosis (F0) and those with advanced Fibrosis (F3) with AUROC, sensitivity and specificity of 0.97, 88% and 100% respectively [18]. These findings were much higher than the findings we found in the present study.
APRI score was assessed in several studies on adult patients with chronic HCV infection with AUROC ranges from 0.77 to 0.94 to detect cirrhosis this higher cut off may be due to the duration and the severity of liver disease and is related to the AST and platelet count reference values [19]. Also this may be because platelet counts decrease and AST levels increase with the progression of liver fibrosis. Platelet generation diminishes secondary to a decreased production of thrombopoietin by hepatocytes [20]. Also, platelets are sequestered and destructed in the spleen as liver fibrosis advances and portal hypertension develops with age [21]. As to AST, ongoing liver injury increases its release from mitochondria, and fibrosis decreases its clearance [22,23].
Conclusion
In children, APRI score and LSM perform reasonably well in fibrosis assessment as they differentiate between no or insignificant fibrosis and significant fibrosis. The routine use of fibroscan and APRI score may decrease the number of liver biopsies performed and they may provide a reliable method of noninvasive monitoring of liver disease progression in children.
References
- Emer F, Alberto Q, Sunitha V, et al. Transient elastography is a useful noninvasive tool for the evaluation of fibrosis in paediatric chronic liver disease. JPGN 2013; 56:72-76.
- The French METAVIR Cooperative Study Group 1994 .Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C.Hepatology1994;20:15-20.
- Westin J, Lagging LM, Wejstal R, et al. Interobserver study of liver histopathology using the Ishak score in patients with chronic hepatitis C infection. Liver 1999;19:183-187.
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97: 2614-2618.
- Ledinghen V, Le BB, Rebouissoux L, et al. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J PediatrGastroenterol Nut2007;45: 443-450.
- Nobili V, Vizzutti F, Arena U, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology 2008;48: 442-448.
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transientelastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007;56:968-973.
- Arena U, Vizzutti F, Abraldes JG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 2008;57:1288-1293.
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518-526.
- Talwalkar JA, Kurtz DM, Schoenleber SJ, et al. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and metaanalysis.ClinGastroenterolHepatol2007; 5: 1214-1220.
- Rockey DC. Noninvasive assessment of liver fibrosis and portal hypertension with transient elastography. Gastroenterology 2008; 134: 8-14.
- Bedossa P, Poynard T :An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology;1996; 24: 289-293.
- Castera L, Forns X, Alberti A: Non-invasive evaluation of liver fibrosis using transient elastography.J Hepato 2008; 48: 835-847.
- Bonnard P,Elsharkawy A,Zalata K, et al. Comparison of liver biopsy and noninvasive techniques for liver fibrosis assessment in patients infected with HCV-genotype 4 in Egypt. Journal of Viral Hepatitis 2015; 22: 245-253.
- Castera L. Prospective comparison of transient elastography, Fibrotest,APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128: 343-350.
- Roxana S, Ioan S, Simona B, et al. A Comparative Study of Non-Invasive Methods for Fibrosis Assessment in Chronic HCV Infection. Hepatitis Monthly 2010; 10: 88-94.
- Dilshad Ahmad Khan, Fatima-Tuz-Zuhra, Farooq Ahmad Khan, et al.Evaluation of diagnostic accuracy of APRI for prediction of fibrosis in hepatitis C patients. J Ayub Med Coll Abbottabad 2008;20: 122-126.
- Judith FC, Segundo MV, Guillermo RG, et al. Non-invasive markers of liver fibrosis in chronic liver disease in a group of Mexican children. A multicenter study.Annals of Hepatology 2012; 11:364-368.
- Sebastiani G, Vario A, Guido M, et al. Stepwise combination algorithms of non-invasive markers to diagnose significant fibrosis in chronic hepatitis C. J Hepatol 2006;44:686-693.
- Adinolfi LE, Giordano MG, Andreana A, et al. Hepatic fibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br J Haematol 2001; 113: 590-595.
- Aster R.: Pooling of platelets in the spleen: role in the pathogenesis of hypersplenic thrombocytopenia.J Clin Invest 1996; 45: 645-657.
- NalpasB,Vassault A,Le Guillou A, et al. Serum activity of mitochondrial aspartate aminotransferase:a sensitive marker of alcoholism with or without alcoholic hepatitis.Hepatology 1984; 4: 893-896.
- Kamimoto Y, Horiuchi S, Tanase S, et al. Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology 1985; 5: 367-375.