- Journal of RNA and Genomics (2011) Volume 7, Issue 1
Non-cell-autonomous RNA interference in mammalian cells: Implications for in vivo cell-based RNAi delivery
| Hannah C Cohen and May P Xiong* Pharmaceutical Sciences Division, University of Wisconsin-Madison, 777 Highland Avenue, Madison, WI 53705, USA |
| *Correspondence to: May Xiong, Email: mpxiong@pharmacy.wisc.edu, Tel: +608 890 0699, Fax: +608 262 5345 |
| Received; 18 October 2011, Revised; 01 November 2011, Accepted; 09 November 2011, Published online; 01 December 2011 |
| © Copyright The Authors: This is an open access article, published under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/2.0/uk/). This license permits noncommercial use, distribution and reproduction of the article, provided the original work is appropriately acknowledged with correct citation details. |
Abstract
RNA interference (RNAi) is a post-transcriptional pathway in which double-stranded RNA (dsRNA) triggers the degradation of complementary mRNA in the cytoplasm of eukaryotic cells. In plants and in some animals, including Caenorhabditis elegans, initiation of RNAi in one cell can lead to sequence-specific RNA silencing in another cell, a phenomenon referred to as non-cell-autonomous RNAi. Until recently, this phenomenon had not been observed in mammalian cells. Here, we review emerging data demonstrating that non-cell-autonomous RNAi occurs in cultured mammalian cells. We discuss possible mechanisms for the transfer of RNAi between mammalian cells and highlight the implications of this phenomenon for the development of in vivo cell-based RNAi delivery.
Keywords |
| RNAi, siRNA, miRNA, non-cell-autonomous RNAi, systemic RNAi, RNAi delivery, cellbased delivery |
Introduction |
| RNA interference (RNAi) is a post-transcriptional pathway in which double-stranded RNA (dsRNA) triggers the degradation of complementary mRNA in the cytoplasm of eukaryotic cells (Fire et al, 1998). In this pathway, the ribonuclease III enzyme Dicer cleaves long dsRNA (Bernstein et al, 2001) into ~21-25-nucleotide (nt) dsRNA duplexes (Hammond et al, 2000; Zamore et al, 2000) referred to as short hairpin RNAs (shRNAs), microRNAs (miRNAs) or short interfering RNAs (siRNAs). (See Figure 1 for more detail regarding when each term is used). The resulting dsRNA duplex is incorporated into the RNA-induced silencing complex (RISC) (Hammond et al, 2000), where helicase enzymes unwind the duplex and the catalytic protein Argonaute 2 (Ago2) cleaves the sense strand of RNA (Matranga et al, 2005). Guided by the antisense strand of RNA (Martinez et al, 2002), the activated RISC targets complementary mRNAs in the cytoplasm for translational repression, mRNA degradation, or cleavage by Ago2 (Liu et al, 2004; Rand et al, 2004; reviewed in Fabian et al, 2010). |
| In plants and in some animals, including Caenorhabditis elegans, RNAi can spread intercellularly (reviewed in Mlotshwa et al, 2002; Voinnet, 2005; Jose and Hunter, 2007; Dinger et al, 2008; Kalantidis et al, 2008; Chitwood and Timmermans, 2010), a phenomenon referred to as non-cell-autonomous RNAi. In plants, RNAi spreads locally from cell-to-cell through plasmodesmata (Himber et al, 2003), which connect the cytosols of adjacent plant cells. RNAi also spreads systemically through the phloem system of the plant (Voinnet et al, 1998; Himber et al, 2003). In C. elegans, injection of dsRNA into the pseudocoelomic body cavity or gonad triggers RNAi in somatic tissues (Fire et al, 1998). Additionally, feeding C. elegans with dsRNA or dsRNA-expressing bacteria induces systemic RNAi (Timmons and Fire, 1998). |
| Despite numerous reports of non-cell-autonomous RNAi in plants and in some animals, until recently, this phenomenon had not been observed in mammalian cells. Here, we review emerging data demonstrating the transfer of RNAi between cultured mammalian cells by both cell contact-independent and cell contact-dependent transfer mechanisms. The existence of non-cell-autonomous RNAi in mammalian cells has important implications for the development of in vivo cell-based RNAi delivery. |
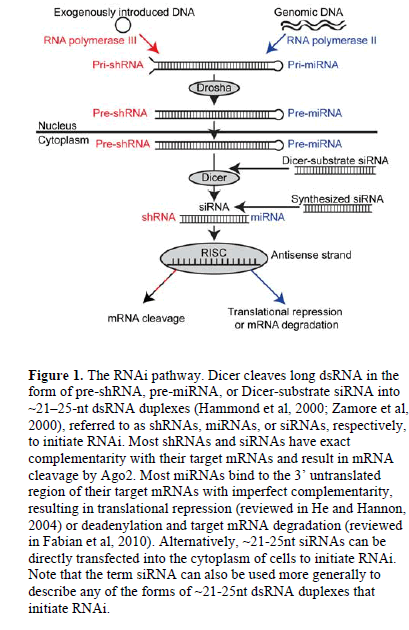 |
| MECHANISMS OF CELL CONTACT INDEPENDENT NON-CELL-AUTONOMOUS RNAi |
| Mechanisms of Cell Contact Independent Non-Cell-Autonomous RNAi |
| Microvesicles |
| Microvesicles (MVs) are plasma membrane fragments varying in size (0.1-1μm in diameter), shape, and composition that are shed from a variety of healthy or damaged cells during plasma membrane blebbing. MVs are thought to mediate intercellular communication by delivering proteins, RNAs, and other cellular cargos from one cell to another (reviewed in Ratajczak et al, 2006b; Cocucci et al, 2009). In agreement, Ratajczak et al (2006a) showed that MVs derived from embryonic stem cells (ESCMVs) delivered embryonic stem cell-derived mRNA into hematopoietic progenitor cells (HPCs), resulting in target protein production and reprogramming of the HPCs. |
| Furthermore, MVs derived from a variety of cell types contain miRNAs (Hunter et al, 2008; Yuan et al, 2009; Collino et al, 2010), suggesting that MVs may shuttle miRNAs between cells. Interestingly, Collino et al (2010) detected certain miRNAs in MVs, but not in their parental cells, suggesting that such miRNAs are selectively packaged in MVs for non-cell-autonomous RNAi. |
| In further supporting the hypothesis that MVs shuttle miRNAs between cells, Yuan et al (2009) showed that ESCMVs fused with co-incubated mouse embryonic fibroblasts to deliver miRNAs. Additionally, Collino et al (2010) demonstrated that miRNA-containing MVs released from donor human bone marrow-derived mesenchymal stem cells (MSCs) delivered miRNAs into co-incubated recipient cells, resulting in target-specific reduction in protein levels. These data demonstrate that certain cell types release MVs that can shuttle functional miRNAs between cells (Figure 2A). |
| Exosomes |
| Also involved in intercellular communication, exosomes are small, 30-100nm in diameter, membrane-bound intraluminal vesicles (ILVs) that are secreted by most cells. Formed during the endolysosomal pathway by the invagination of the limiting endosomal membrane in multivesicular bodies (MVBs), exosomes are released into the extracellular space when MVBs fuse with the plasma membrane. Exosomes contain cytosolic and plasma membrane proteins and express cell recognition molecules that allow for selective cellular uptake (reviewed in Théry et al, 2002; Simons and Raposo, 2009; Mincheva-Nilsson and Baranov, 2010). Similarly, secretory exosomes containing RNAs and proteins may be a major mode of communication within the nervous system (reviewed in Smalheiser, 2007). Exosomes secreted from a variety of cell types contain miRNAs (Valadi et al, 2007; Skog et al, 2008; Taylor and Gercel-Taylor, 2008; Luo et al, 2009; Kosaka et al, 2010; Michael et al, 2010; Ohshima et al, 2010; Pegtel et al, 2010; Zomer et al, 2010; DeIuliis et al, 2011; Levänen et al, 2011; Mittelbrunn et al, 2011), leading to the name “exosomal shuttle RNA” (Lotvall and Valadi, 2007; Valadi et al, 2007). In fact, certain miRNAs exist in higher levels in exosomes than in their parental cells (Valadi et al, 2007; Ohshima et al, 2010; DeIuliis et al, 2011; Mittelbrunn et al, 2011), suggesting that such miRNAs are selectively packaged into MVBs and secreted as exosomes for non-cell-autonomous RNAi. |
| Pegtel et al (2010) has provided further evidence to support the hypothesis that exosomes shuttle miRNAs between cells by demonstrating that Epstein-Barr virus (EBV)-infected B cells secrete exosomes containing EBVencoded miRNAs, which knocked down EBV target genes in co-incubated monocyte-derived dendritic cells. Similarly, Kosaka et al (2010) showed that cultured HEK293 human embryonic kidney cells and COS-7 African green monkey kidney fibroblast-like cells secrete miRNA-containing exosomes, which were internalized in recipient cells leading to sequence-specific RNA silencing. These data demonstrate that certain cell types release exosomes that can shuttle functional miRNAs between cells (Figure 2B). |
| Apoptotic bodies, miRNA-Ago2 complexes and SIDT-1 In addition to MVs and exosomes, apoptotic bodies – a form of MVs released during programmed cell death – may transport miRNAs between cells (Figure 2C). Zernecke et al (2009) demonstrated that apoptotic bodies isolated from cultured endothelial cells could deliver miRNA-126 into recipient vascular cells, inducing the production of chemokine CXC motif ligand 12 (CXCL12). Alternatively, miRNAs were shown to exist extracellularly in human plasma as vesicle-free Ago2 complexes (Arroyo et al, 2011). Because Ago2 is the catalytic component of RISC (Liu et al, 2004; Rand et al, 2004), these miRNAAgo2 complexes could be responsible for inducing RNAi in recipient cells (Figure 2D). |
| SID-1 (systemic RNAi-defective-1) is a protein channel that is necessary for the import of dsRNA into most cells in C. elegans (Winston et al, 2002; Feinberg and Hunter, 2003). Overexpression of the mammalian homologue of SID-1 (i.e., SIDT-1) was shown to increase internalization of siRNA and RNAi in mammalian cells soaked in siRNA-containing medium (Duxbury et al, 2005; Tsang et al, 2007) (Figure 2E). However, at endogenous levels, SIDT-1 expression appeared to be insufficient for siRNA uptake into mammalian cells (Tsang et al, 2007). |
Mechansims of Cell Contact-Deependent Non-cell-Autonomous RNAi |
| Tunneling nanotubes |
| Tunneling nanotubes (TNTs) were first identified in cultured rat pheochromocytoma PC12 cells as intercellular structures with diameters of 50-200nm and lengths of up to several cell diameters (Rustom et al, 2004). They were shown to hover between cells, transferring membrane components and organelles between the cytosols of adjoining cells (Rustom et al, 2004). TNTs with a variety of diameters, lengths, and compositions have since been identified in vitro in numerous cell types (reviewed in Gerdes and Carvalho, 2008; Gurke et al, 2008) and in vivo between bone marrow-derived MHC class II-positive cells in the corneal stroma of mice (Chinnery et al, 2008). Figure |
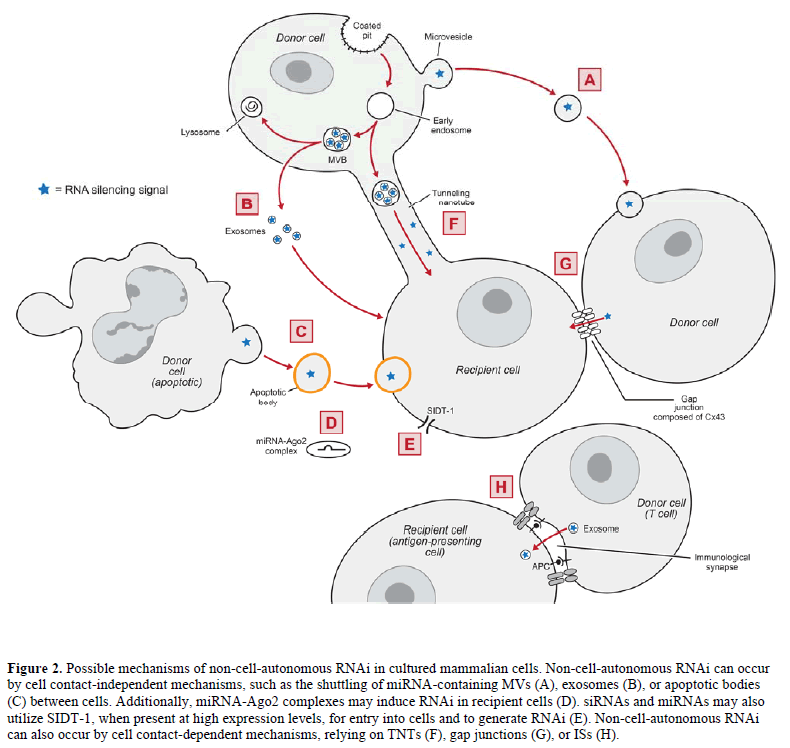 |
| Two types of TNTs have been identified in cultured human macrophages: TNTs less than 0.7μm in diameter that contain F-actin, and TNTs greater than 0.7μm in diameter that contain F-actin and microtubules (Önfelt et al, 2006). Bidirectional transfer of mitochondria and intracellular vesicles (including late endosomes and lysosomes) can occur in the latter variety of TNTs (Önfelt et al, 2006), suggesting that TNTs could mediate the transfer of miRNA-containing endosomal vesicles between cells (Figure 2F). In agreement, Belting and Wittrup (2008) reviewed TNTs as a potential mechanism for the intercellular transfer of genetic material, due in part to their ability to transfer endosomal vesicles between cells. Additionally, TNTs are remarkably similar to plasmodesmata (reviewed in Rustom, 2009), which are involved in the transfer of siRNAs between plant cells. In fact, donor human MSCs were shown to infuse siRNAs into co-cultured recipient neural progenitor cells (Mitchell et al, 2011; Olson et al, 2011) through TNTs and other mechanisms (Nolta JA, personal communication). |
| Gap junctions |
| Gap junctions are intercellular channels that span the plasma membranes of adjoining cells, connecting the cytosols and allowing for cell-to-cell communication (reviewed in Bruzzone et al, 1996). In a gap junction, each of two adjacent cells contains a connexon, which is a hemichannel composed of six connexin (Cx) proteins. Gap junctions form from two identical connexons (homotypic) or two different connexons (heterotypic). Most tissues express more than one type of connexin and connexins often have distinct tissue and cellular distributions. Gap junction intercellular communication (GJIC) allows watersoluble molecules (e.g., ions, second messengers, and small metabolites) to diffuse between cells (reviewed in Bruzzone et al, 1996). Emerging evidence suggests that GJIC also mediates the transfer of RNAi between cells. |
| Valiunas et al (2005) demonstrated that there was transfer of RNAi targeting DNA polymerase β from donor normal rat kidney (NRK) cells stably expressing shRNA against DNA polymerase β to co-cultured recipient NRK cells (Valiunas et al, 2005). NRK cells express Cx43 (Hand et al, 2002); however, repeated experiments using donor and recipient cells that expressed Cx26 and Cx32 but not Cx43, or that were connexin-deficient, did not show knockdown of DNA polymerase β in recipient cells (Valiunas et al, 2005). |
| In similar co-culture experiments, Wolvetang et al (2007) demonstrated a gap junction (Cx43 and/or Cx45)-mediated transfer of RNAi targeting green fluorescent protein (GFP) from donor human embryonic stem cells (hESCs) stably expressing shRNA against GFP to co-cultured recipient hESCs stably expressing GFP. Likewise, Kizana et al (2009) observed the transfer of RNAi targeting enhanced GFP (eGFP) from donor neonatal rat ventricular myocytes (NRVMs) stably expressing shRNA against eGFP to cocultured recipient NRVMs stably expressing eGFP. NRVMs express Cx43 (Kizana et al, 2007); however, there was no change in eGFP levels when the NRVMs expressed a Cx43 dominant-negative mutant, suggesting that the reduction in eGFP was dependent on Cx43 gap junctions (Kizana et al, 2009). Lastly, Lim et al (2011) reported that miRNAs against CXCL12 were transferred from donor bone marrow stromal cells to co-cultured recipient breast cancer cells through gap junctions composed of Cx43, leading to reduced expression of CXCL12 in the recipient cells. Together, these studies demonstrate that RNAi transfers between cells through gap junctions composed of certain connexins, including Cx43 (Figure 2G). |
| Immunological synapses |
| An immunological synapse (IS) is a junction that forms at the interface of a T cell and an antigen-presenting cell (APC), allowing for cell-to-cell interactions to modulate the immune response (reviewed in Rodríguez- Fernández et al, 2010). Mittelbrunn et al (2011) demonstrated that miRNA-containing exosomes transferred from T cells to APCs upon antigen-induced IS formation, resulting in sequence-specific RNA silencing in the recipient APCs. The T cell MVBs polarized towards the IS, which enhanced the secretion of miRNA-containing exosomes (Mittelbrunn et al, 2011). These data suggest that RNAi may transfer between immune cells through ISs (Figure 2H). |
Considerations In Studies of Non-Cellautonomus RNAi in Mammallan Cells |
| The method of introducing the RNAi agent into the donor cells |
| Different methods of introducing the RNAi agent into the donor cells may impact the transfer of RNAi between cells. For example, a common method of transfecting cells with siRNA involves complexing the siRNA with a cationic lipid carrier to create siRNA-lipoplexes. Recent data suggest that a majority of siRNA-lipoplexes introduced into cells persist in endolysosomes (Lu et al, 2009). Because endolysosomal trafficking may be involved in intercellular transfer of RNAi (reviewed in Gibbings and Voinnet, 2010), siRNA-lipoplexes within endolysomes may be more likely to transfer between cells than siRNA introduced into cells by a different method. |
| The method of introducing the RNAi agent into the donor cells may also impact the potency of RNA silencing in the recipient cells. Assuming that the donor cells remain viable at the target site, donor cells stably expressing shRNA or miRNA may induce more potent RNA silencing in the recipient cells than donor cells transiently expressing siRNA. |
| Donor to recipient cell ratio |
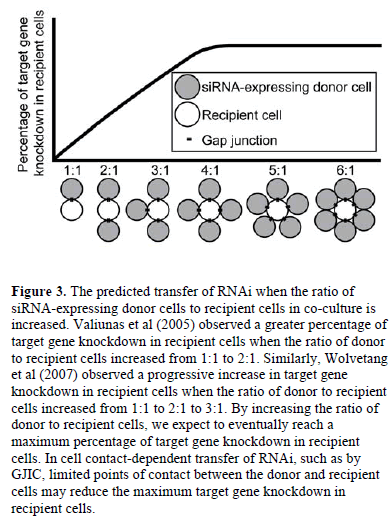
| Increasing the ratio of siRNA-expressing donor cells to recipient cells in co-culture increased target gene knockdown in recipient cells (Valiunas et al, 2005; Wolvetang et al, 2007), suggesting that a higher ratio of donor to recipient cells results in more RNA silencing signals entering the recipient cells. By increasing the ratio of donor to recipient cells, we expect to eventually reach a maximum percentage of target gene knockdown in recipient cells (Figure 3). This maximum would depend on a variety of factors including the rate of target mRNA turnover in recipient cells, the number of RNA silencing signals transferred by each donor cell, and the potency of each RNA silencing signal. Additionally, in cell contactdependent RNAi transfer, limited points of contact between the donor and recipient cells may increase the time of co-culture required to reach the maximum target gene knockdown in recipient cells or may reduce the maximum altogether. |
| RNAi in donor cells following intercellular transfer of RNAi to recipient cells |
| While the transfer of RNA silencing signals from donor to recipient cells may lessen the potency of RNAi in the donor cells, we predict that the transfer would not eliminate RNAi in the donor cells. Each activated RISC undergoes multiple rounds of RNA silencing (Hutvágner and Zamore, 2002), suggesting that potent RNA silencing could occur in the donor cells despite the transfer of some RNA silencing signals to the recipient cells. Additionally, an RNA-dependent RNA polymerase (RdRP) has recently been identified in mammalian cells (Maida et al, 2009). |
 |
| RdRPs are primed by existing siRNAs to produce more dsRNAs, thereby amplifying RNA silencing (Sijen et al, 2001; reviewed in Nishikura, 2001). Thus, the transfer of RNA silencing signals from donor to recipient cells would not necessarily eliminate RNAi in the donor cells. Unfortunately, limited understanding of the molecular nature of the transferred RNA silencing signal makes it difficult to predict how RdRPs or the multi-turnover nature of RISC impacts the transfer of RNAi between mammalian cells. |
Mechanistic Insights into Non-cellautonomous RNAi in Mammallan Cells |
| Molecular nature of the transferred RNA silencing signal |
| In plants, the RNA silencing signal that spreads short distances (10-15 cells) from cell to cell through plasmodesmata is a 21-nt siRNA duplex (Himber et al, 2003; Dunoyer et al, 2005; Dunoyer et al, 2010; reviewed in Chitwood and Timmermans, 2010). However, the molecular nature of the RNA silencing signal that spreads systemically in plants and in C. elegans remains unclear (reviewed in Mlotshwa et al, 2002), as does the molecular nature of the transferred RNA silencing signal in mammalian cells. |
| It is unlikely that long dsRNA is the RNA silencing signal that transfers between mammalian cells, as dsRNAs longer than 30nt activate the interferon system (Minks et al, 1979; Manche et al, 1992). In agreement, Valiunas et al (2005), Wolvetang et al (2007), and Kizana et al (2009) hypothesized that Dicer-processed shRNA was the RNA silencing signal transferred between mammalian cells through gap junctions. Valiunas et al (2005) demonstrated that oligonucleotides (morphilinos) simulating siRNAs, with molecular weights of ~2-4kDa, minor diameters of 1.0-1.1nm, and lengths of 7.6nm could diffuse between cells through Cx43 gap junctions. The oligonucleotide permeation through the Cx43 gap junctions decreased as the length of the oligonucleotides increased, which was expected as the minor diameter of the oligonucleotides was close to the pore diameter of the gap junctions (~1.0-1.5nm) (Valiunas et al, 2005). A hybridized, double-stranded 12- mer oligonucleotide had significantly decreased permeation through the Cx43 gap junctions when compared with a single-stranded 12-mer oligonucleotide, suggesting that single-stranded siRNAs are more likely to transfer through Cx43 gap junctions than hybridized siRNAs (Valiunas et al, 2005). Additionally, Kizana et al (2009) determined that, compared with shRNAexpressing donor cells, co-cultured recipient cells contained 25% of the copy number of the antisense strand of shRNA, suggesting that the antisense strand of shRNA, or possibly the shRNA duplex, transfers from donor to recipient cells through Cx43 gap junctions. |
| Rechavi et al (2009) demonstrated the transfer of 22-nt Cy3-labeled dsRNAs, or possibly metabolic products of those dsRNAs, from donor B cells to recipient T cells. However, 22-nt FITC-conjugated locked nucleic acids did not transfer between cells, suggesting that structural specificity is involved in intercellular RNA transfer (Rechavi et al, 2009). Additionally, Rechavi et al (2009) did not detect the movement of the RISC component Ago2 between donor and recipient cells, suggesting that small RNAs transfer between cells independently of Ago2. Based on these studies, we hypothesize that the antisense strand of Dicer-processed siRNA may be the RNA silencing signal that transfers between cells in non-cellautonomous RNAi; however, we recognize that there may be different RNA silencing signals depending on the mechanism of non-cell-autonomous RNAi. |
| Endolysosmal trafficking and intercellular transfer of RNAi |
| There is increasing data linking RNAi to endolysosomal trafficking (reviewed in Siomi and Siomi, 2009; Gibbings and Voinnet, 2010). In the endolysosomal pathway, the endosomal sorting complex required for transport (ESCRT) is required for the invagination of the limiting endosomal membrane in MVBs to form ILVs (reviewed in Babst, 2005). ESCRT is also required for the sorting of endosomal cargo proteins into ILVs. MVBs can fuse with the lysosome for degradation or they can fuse with the plasma membrane to secrete their ILVs, which are then referred to as exosomes. |
| Gibbings et al (2009) demonstrated that RISC proteins GW182 and Ago2 were present with miRNAs in endosomes/MVBs from cultured monocytes, suggesting that miRNAs and RISC congregate on endosomes and/or MVBs. Furthermore, Lee et al (2009) discovered that in cultured HeLa cervical cancer cells, blocking the maturation of MVBs into lysosomes with Hermansky- Pudlak Syndrome 4 mutants stimulated RNAi. These findings, together with data showing that exosomes contain miRNAs (Valadi et al, 2007; Skog et al, 2008; Taylor and Gercel-Taylor, 2008; Luo et al, 2009; Kosaka et al, 2010; Michael et al, 2010; Ohshima et al, 2010; Pegtel et al, 2010; Zomer et al, 2010; DeIuliis et al, 2011; Levänen et al, 2011; Mittelbrunn et al, 2011), suggest that molecular pathways control the packaging of miRNA and RISC components into endosomes/MVBs and mediate intercellular transfer of RNAi (reviewed in Siomi and Siomi, 2009; Gibbings and Voinnet, 2010). However, the details of such pathways remain unclear. Although the ESCRT machinery appears to be involved in MVB formation (reviewed in Babst, 2005), evidence suggests that sphingomyelinase 2, which regulates ceramide biosynthesis, but not the ESCRT machinery, is involved in the secretion of miRNA-containing exosomes (Kosaka et al, 2010; Mittelbrunn et al, 2011). |
Implications for In vivo Cell-Based RNAi Delivery |
| Despite the enormous potential of RNAi for disease therapy, current in vivo RNAi delivery strategies (reviewed in Whitehead et al, 2009; Lares et al, 2010), such as those using liposomes, cationic polymers, or viral vectors, have been hindered by a variety of challenges (reviewed in Trehan et al, 2010), including immune system activation and inefficient cell targeting. Cell-based RNAi delivery, in which donor cells act as RNAi delivery vehicles, can potentially overcome these challenges (reviewed in Brink et al, 2010; Brink et al, 2011). Autologous or immunoprivileged allogeneic donor cells may avoid host immune system activation, and certain cell types inherently migrate to tumors or wounds. Furthermore, in areas where traditional RNAi delivery vehicles have limited diffusion, the spread of RNAi may be more efficient by transferring the RNA silencing signal from cell to cell. |
| MSCs are excellent candidates for in vivo cell-based RNAi delivery because they exhibit innate tumor- and woundhoming abilities, are considered immunoprivileged, and can be isolated in large numbers from bone marrow or adipose tissue (reviewed in Brink et al, 2010; Dwyer et al, 2010, Hu et al, 2010). Furthermore, MSCs express Cx43 (Valiunas et al, 2004), allowing for the transfer of RNAi from MSCs to Cx43-expressing recipient cells by GJIC. MSCs can also form TNTs (Plotnikov et al, 2010), and release miRNA-containing MVs (Collino et al, 2010) and exosomes (DeIuliis et al, 2011), illustrating their potential to participate in non-cell-autonomous RNAi by multiple mechanisms. |
Conclusions |
| Emerging evidence demonstrates that non-cellautonomous RNAi occurs in cultured mammalian cells. This phenomenon can occur by cell contact-independent mechanisms (Figure 2A-D), such as the shuttling of miRNA-containing MVs, exosomes, or apoptotic bodies between cells. Non-cell-autonomous RNAi can also occur by cell contact-dependent mechanisms, relying on TNTs, gap junctions, or ISs (Figure 2F-H). Non-cell-autonomous RNAi in mammalian cells may allow for the development of in vivo cell-based RNAi delivery, which has the potential to overcome major challenges in RNAi delivery and allow for effective RNAi therapies. |
| Acknolwledgements |
| We would like to acknowledge Sally Griffith-Oh at the University of Wisconsin-Madison for help in creating Figure 2. This work was supported with start-up funds from the University of Wisconsin-Madison School of Pharmacy and through a Department of Defense (DoD) National Defense Science and Engineering Graduate (NDSEG) Fellowship to HC Cohen. |
Statements of Competing Interests |
| None declared. |
List of Abbreviations |
| Ago2; Argonaute 2 |
| APC; antigen-presenting cell |
| Cx; connexin |
| CXCL12; chemokine CXC motif ligand 12 |
| EBV; Epstein-Barr virus |
| eGFP; enhanced GFP |
| ESCMVs; embryonic stem cell-derived MVs HPCs; hematopoietic progenitor cells |
| ESCRT; endosomal sorting complex required for transport |
| GFP; green fluorescent protein |
| GJIC; gap junction intercellular communication, NRK; normal rat kidney |
| hESCs; human embryonic stem cells |
| ILVs; intraluminal vesicles |
| IS; immunological synapse |
| MSCs; mesenchymal stem cells |
| MVBs; multivesicular bodies |
| MVs; microvesicles |
| NRVMs; neonatal rat ventricular myocytes |
| RdRP; RNA-dependent RNA polymerase |
| RISC; RNA-induced silencing complex |
| SID-1, systemic RNAi-defective- |
| TNTs; tunneling nanotubes |
References
|