Case Report - Journal of Cancer Immunology & Therapy (2019) Volume 2, Issue 1
Nocardiosis in cancer patients
Goswami Parijat N1*, Panchal Harsha2, Parikh Sonia2, Rajpara Hitesh3 and Hemang Purohit1
1Department of Microbiology, Gujarat Cancer and Research Institute, NCHC, Asarwa, Ahmedabad, India
2Medical Oncology, Gujarat Cancer and Research Institute NCHC, Asarwa, Ahmedabad, India
3Department of Radiology, Gujarat Cancer and Research Institute NCHC, Asarwa, Ahmedabad, India
- *Corresponding Author:
- Parijat N Goswami
Professor and Head of Microbiology
Division of Microbiology
Gujarat Cancer and Research Institute
Asarwa, Ahmedabad, India
Tel: +9327053741
E-mail: bannuhyd@yahoo.co.in
Accepted date: January 03, 2019
Citation: Goswami PN, Panchal H, Parikh S, et al. Nocardiosis in cancer patients. J Cancer Immunol Ther. 2019;2(1):1-3.
Abstract
Myelofibrosis is a serious bone marrow disorder that disrupts body's normal production of blood cells. The result is extensive scarring in bone marrow, leading to severe anaemia, weakness, fatigue and often an enlarged spleen. Myelofibrosis is an uncommon type of chronic leukaemia, a cancer that affects the blood-forming tissues in the body. It belongs to a group of diseases called myeloproliferative disorders. Many people with Myelofibrosis get progressively worse, and some may eventually develop a more serious form of leukaemia. Yet it's also possible to have Myelofibrosis and live symptom-free for years. Treatment for Myelofibrosis, which focuses on relieving symptoms, can involve a variety of options. Infections due to Nocardia spp are rare; localized skin forms are often seen among categories with limited exposure, such as agricultural workers. Although uncommon, disseminated infections are severe and life-threatening and affect mainly immunodeficient patients. The most common localization is the lung, but Central Nervous System (CNS), soft tissue, blood, and lymph nodes are also involved in disseminated Nocardiosis.Keywords
Myelofibrosis, Nocardiosis, Immunocompromised patients
Introduction
Myelofibrosis is a serious bone marrow disorder that disrupts body's normal production of blood cells. The result is extensive scarring in bone marrow, leading to severe anaemia, weakness, fatigue and often an enlarged spleen. Myelofibrosis is an uncommon type of chronic leukaemia, a cancer that affects the blood-forming tissues in the body. It belongs to a group of diseases called myeloproliferative disorders. Many people with Myelofibrosis get progressively worse, and some may eventually develop a more serious form of leukaemia. Yet it's also possible to have Myelofibrosis and live symptom-free for years. Treatment for Myelofibrosis, which focuses on relieving symptoms, can involve a variety of options [1].
Infections due to Nocardia spp are rare; localized skin forms are often seen among categories with limited exposure, such as agricultural workers. Although uncommon, disseminated infections are severe and life-threatening and affect mainly immunodeficient patients. The most common localization is the lung, but Central Nervous System (CNS), soft tissue, blood, and lymph nodes are also involved in disseminated Nocardiosis [2].
Case Report
Nineteen years old patient suffering with low grade fever since 15 days was admitted in GCRI on 9th May 2018 for further investigations and treatment. On examination patient had no history of cancer, no history of diabetes or hypertension or any other addictions. On examination patient was pale. Investigations like complete blood picture showed 8.5% hemoglobin, 1200 cu mm WBCs and 44000 Platelets. Peripheral blood showed 3% blast cells. The provisional diagnosis made was Myelodysplastic syndrome (MDS) by the clinician. Patient had fluctuating fever. He was given antipyretic and antibiotics for treating fever. Blood culture and other cultures were advised. They were normal. Simultaneously bone marrow examination diagnosed Myelofibrosis on next day by the pathologist. Patient’s other reports of HIV, HBsAg and HCV were Non-Reactive and Negative. His initial Chest X-Ray showed presence of consolidation. His 2D Echo pictures showed normal LV size and fair LV Function, reduced LV compliance. Other haematological investigations of TLC, DC was normal except Hemoglobin which is persistently low compared to normal limit ~7 g. Serum electrolytes were within normal limits. Notably on same date patients WBC were also increased to 1.6 × 103 and later it was normal in follow up reports. Patient’s Serum creatinine and electrolytes were also almost normal. Antibiotic therapy given to him was Piperacillin, Tazobactum, Meropenem, Amikacin, Linezolid, Levofloxacin and Fluconazole along with antacid, paracetamol during his admission and then on his recovery he was discharged with follow up. As per records patient was lost to Follow up (LFU) for around 5 months.
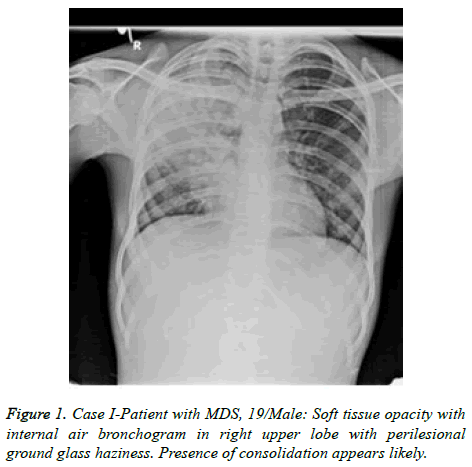
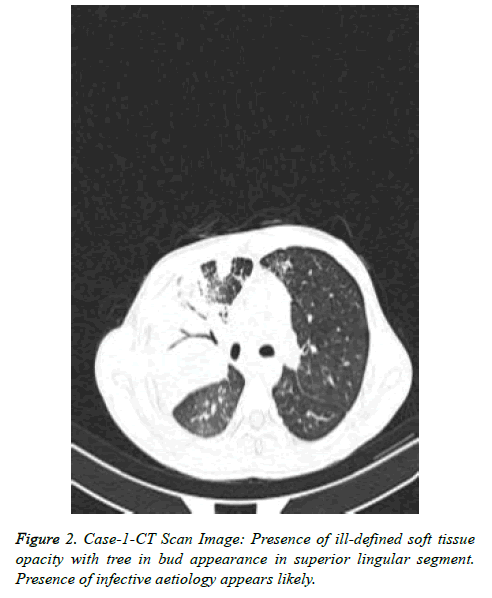
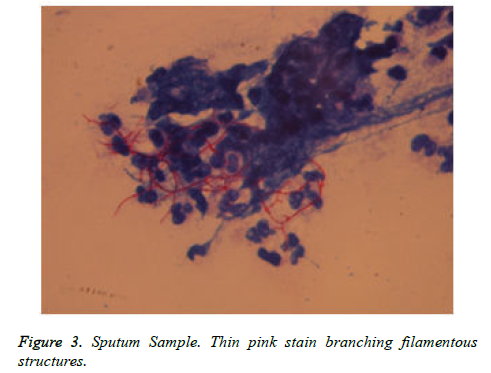
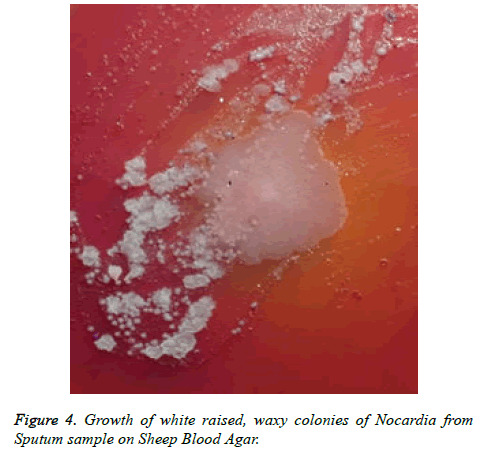
Patient got readmitted in June 2018 with presenting complains of cough, expectoration and fever presenting as lower respiratory tract symptoms and he was again subjected for investigations to rule out any infections. CBC, Total count and X-Chest were advised. He was given symptomatic treatment to relieve respiratory symptoms. Patient was later given i.e. Piperacillin/tazobactum, levofloxacillin and he was not responding to these antibiotics. Further X-Ray chest showed consolidation in right upper and lower lungs (Figure 1). CT scan showed picture of infection (Figure 2). Sputum sample was resend to microbiology. Microscopy showed thin branching gram positive branching structures. Modified Kinyoun’s acid fast stain (Cold method) was performed and microscopy showed thin branching structures broken in continuity resembling Nocardia under oil immersion (Figure 3). Since it grows slowly Nocardia is often overgrown by normal flora and other bacteria. On Blood agar, growth of colonies appeared on the 4th day of incubation. They appeared white, velvety and waxy raised from the surface of blood agar plate (Figure 4). Nocardia species are classically grampositive, strictly aerobic, filamentous, branching, weakly acidfast bacilli. They may be isolated on routine bacterial, fungal, and mycobacterial media. Colonies may appear within 4 days, but may require up to 2-4 weeks of culture. Pre-treatment of the patient with antibiotics that slow but do not kill Nocardia will most often increase the time required to grow Nocardia from clinical isolates. If Nocardiosis is suspected clinically, the bacteriology laboratory should be informed and cultures should be kept longer than usual. Nocardia can also be difficult to isolate by culture because of overgrowth by faster n-growing on-pathogenic colonizers that may mask its presence [3].
Discussion
Nocardiosis is an opportunistic infection in patients who are chronically ill and is caused by more than 30 species of Nocardiae of human clinical significance, with the majority of isolates being N. nova complex, N. abscessus, N. transvalensis complex, N. farcinica, N. asteroides type VI (N. cyriacigeorgica), and N. brasiliensis. These species cause a wide variety of diseases and have variable drug susceptibilities. Accurate identification often requires referral to a reference laboratory with molecular capabilities, as many newer species are genetically distinct from established species yet have few or no distinguishing phenotypic characteristics. These species cause a wide variety of diseases and have variable drug susceptibilities. Correct identification is important in deciding the clinical relevance of a species and in the clinical management and treatment of patients with nocardial disease.
As it is a known fact that Nocardiae are ubiquitous in the environment and can be found worldwide as saprophytic components in fresh- and saltwater, soil, dust, decaying vegetation, and decaying fecal deposits from animals, health care-associated transmission or acquisition of the nocardia has been documented but is relatively rare.
However, nocardial infections are not considered to be communicable from person to person, although this may relate to the relative infrequency of close association of high-risk patients. After discharge the patient didn’t come for follow-up for five months. He was readmitted with presenting symptoms of lower respiratory tract and was investigated for the aetiology. On taking history it was known that the patient lives in rural area and he landed with pulmonary nocardiosis. He was relieved of the infection as soon as Trimethoprimsulamethoxazole was instituted along with anti-fungal and antibiotics.
In conclusion after diagnosis and treatment the patient is still coming for follow-up regularly. It is important to vigilantly diagnose such patients clinically as well as diagnostically. All the diagnostic elements help in patient support like radiodiagnosis and microbiological diagnosis. Microscopy is the gold standard tool for sensing the presence of such rare organisms. Many laboratories perform selective investigations on request. But in our experience we have seen that performing Kinyoun’s modified acid fast stain for all the respiratory samples is helpful to catch such type of rare organisms. It will be a boon for any diagnostic services when there will be culture positivity. Therefore the diagnosis is time consuming and the clinicians will have to be patient enough for fetching the reports.
References
- Cattaneo C, Antoniazzi F, Caira M, et al. Nocardia spp infections among hematological patients: results of a retrospective multicenter study. Int J Infect Dis. 2013; 17(8): e610-4
- Chedid MB, Chedid MF, Porto NS, et al. Nocardial infections: report of 22 cases. Rev Inst Med Trop Sao Paulo. 2007; 49(4): 239-46.
- Yildiz O, Doganay M. Actinomycoses and Nocardia pulmonary infections. Curr Opin Pulm Med. 2006; 12(3): 228-34.
- Martinez R, Reyes S, Menendez R. Pulmonary nocardiosis: risk factors, clinical features, diagnosis and prognosis. Curr Opin Pulm Med. 2008; 14(3): 219-27.