- Biomedical Research (2014) Volume 25, Issue 3
Nicotinic acid, lauric acid and kaempferol abolish ATP-binding cassette transporter subfamily A member 1 (ABCA1) down-regulation by TNF-? in hepatocarcinoma HepG2 cell line.
Hoay-Yan Cheah, Yew-Yen Wong, Hong-Kin Wong, Wyi-Sian Lim, Choy-Hoong Chew*Department of Biomedical Science, Faculty of Science, Universiti Tunku Abdul Rahman, Jalan Universiti, Bandar Barat, 31900 Kampar, Perak, Malaysia.
- *Corresponding Author:
- Chew Choy Hoong
Department of Biomedical Science
Faculty of Science, Universiti Tunku Abdul Rahman
Jalan Universiti, Bandar Barat
31900 Kampar, Perak, Malaysia
Accepted date: March 20 2014
Abstract
The aim of this study was to investigate the effect of different concentrations of antioxidants on ABCA1 mRNA expression in TNF-α stimulated hepatocarcinoma (HepG2) cell line. TNF- α-stimulated cells were treated with 1 μM, 5 μM, 10 μM and 20 μM of nicotinic acid, lauric acid or kaempferol for 24 h, respectively. Real time RT-PCR was carried out to analyze the expression of the genes. Relative ABCA1 mRNA levels were normalized to beta-actin and their relative expression to untreated cells was analyzed using delta-delta CT method. Here we show that ABCA1 mRNA levels were significantly suppressed by 20 ng/ml of TNF-α, but all three antioxidants were able to antagonize this suppressive effect of TNF-α. High doses (20 μM) of the antioxidants significantly up-regulated the ABCA1 gene expression. This study successfully profiled that all the three antioxidants were able to alleviate the downregulatory effect of TNF-α on ABCA1 transcript level in HepG2 cells. Although stimulation with kaempferol alone induced ABCA1 expression to high levels, its ability to reduce TNF-α inhibitory response was the weakest amongst the three antioxidants.
Keywords
Nicotinic acid, lauric acid, kaempferol, ABCA1, TNF-α, expression
Introduction
The high density lipoproteins (HDLs) are major carriers of cholesterol in the blood stream and it has a protective function against atherosclerotic coronary disease. Besides, it is involved in the process of reverse cholesterol transport (RCT) to regulate cholesterol homeostasis [1]. ATP-binding cassette transporters, ABCA1 and ABCG1, are cell surface transporters that mediate the reverse cholesterol transport (RCT), a process that delivers cholesterol from the peripheral tissue back to the liver for disposal [2]. On the other hand, foam cell homeostasis is regulated by the RCT pathway. ABCA1, a target gene of liver X receptor (LXR), mediates the rate-controlling step in HDL synthesis by increasing cellular efflux of phospholipids and cholesterol to apo A-I containing lipoproteins, and thus plays a protective role in preventing atherosclerosis [3]. ABCA1 and ABCG1 are highly expressed in hepatocytes, which are the major source of circulating plasma HDL [4] and the control of ABCA1 and ABCG1 expression of transcriptional regulatory networks in lipid metabolism is relevant to the development atherosclerosis. In fact, the mutation of ABCA1 leads to the development of Tangier disease, which is characterized by the accumulation of cholesterol ester in reticuloendothelial cell, low serum HDL and increased risk of coronary heart disease [5].
Nicotinic acid, also known as niacin, is a type of Vitamin B3 which occurs naturally in food such as dairy product and legume. Nicotinic acid is important for cell respiration and metabolism of carbohydrate, fat and protein. Furthermore, it is used to treat high levels of cholesterol and triglycerides in the blood in order to improve the blood cholesterol profile [6].
Studies have shown that the treatment of nicotinic acid reduced low-density lipoprotein cholesterol (LDL-C), triglycerides and very low-density lipoprotein (VLDL) and increased HDL-C as well [7-9]. Besides, anti-lipolytic action of nicotinic acid was found to reduce circulating non-esterified fatty acid levels, thereby impairing hepatic triglyceride synthesis and VLDL secretion [10,11]. Thus, the low level of VLDL would lower the plasma cholesterol and phospholipids levels [12].
Lauric acid is a natural saturated fatty acid (SAFA) with 12 carbon atom chain made from plants as an energy reserve. It is a component of triglycerides and make up consist half of the fatty acid content in coconut oil, laurel oil and palm kernel oil. It is claimed to have antimicrobial properties [13]. However, SAFAs have been associated with deleterious effect in atherosclerosis as it reduced triacyglycerols (TAG) levels, low density lipoprotein cholesterol (LDL-C) and total cholesterol [14].
On the other hand, kaempferol is one of the polyphenolic flavonoids that could inhibit growth of human leukaemic cells [15], but also claimed to be able to protect some cancer cell lines [16]. Studies have proven that kaempferol functions either as cancer growth inhibitor or agonist depending on the concentrations used. It was found to enhance cancer cell growth and DNA synthesis at low concentration (1-10 μM), while it inhibited cancer cell growth and DNA synthesis at high concentration (20-90 μM) [17].
As a multifunctional cytokine, TNF-α plays important roles in apoptosis, cell survival, inflammation and immunity. The main function of TNF-α is to induce inflammation via up-regulation of gene transcription through NF-κB signaling pathway [18,19]. At the same time, it could decrease HDL cholesterol levels by attenuating the basolateral efflux of cholesterol to apo A-I and ABCA1 promoter activity which would decrease the expression of ABCA1 [3]. Besides, the production of TNF-α also activates macrophage foam cells which would lead to the maintenance and progression of atherogenesis [2].
It was therefore hypothesized that the antioxidants would alleviate the inhibitory response elicited by the cytokine TNF-α, which has been proven to inhibit the expression of the genes [1,3]. Due to lack of evidences in this aspect, this study was designed to investigate the effects of different concentrations of the antioxidants on either alleviating or amplifying the TNF-α inhibitory response on ABCA1 mRNA expression.
Materials and Methods
Preparation of antioxidants
Nicotinic acid and kaempferol were purchased from CalBiochem, UK and Sigma Aldrich, USA, respectively, and diluted in deionized water to prepare 1 mM stock solution. Lauric acid powder (Merck, USA) was solubilized in pure dimethyl sulfoxide (DMSO) (Labscan, Poland) to constitute a 1 mM (w/v) solution of lauric acid. The samples were stored at -4°C.
Cell culture maintenance
Hepatocellular carcinoma HepG2 cell line obtained from American Type Cell Culture (ATCC, USA) was used in this study and cultured in accordance to Lim et al. [20].
Cell Treatments and Total Cellular RNA Extraction
HepG2 cells were grown until they reached 60-70% confluence in 25 cm2 tissue culture flasks. The medium was discarded and the cells were washed twice with PBS. All flasks were pre-treated with MEM (Gibco, USA) and 0.5% of FBS (Gibco, USA) for 2 h prior to the addition of cytokine. To confirm the effect of TNF-α on the ABCA1 mRNA expression, 20 ng/ml of TNF-α (Chemicon, USA) was added into pretreated tissue cultures and the cells were incubated in the 5% (v/v) CO2 incubator (Binder, Germany) at 37°C for 24 h. Different concentrations of nicotinic acid (1 μM, 5 μM, 10 μM and 20 μM) were then added to the 24-h-cytokine-treated-sample.
As for the untreated sample, the cells were supplemented with fresh MEM and 0.5% (v/v) FBS only without the presence of TNF-α. Another control sample was treated with 20 μM nicotinic acid alone. All cells were then incubated in the 5% (v/v) CO2 incubator at 37°C for 24 h. The experiments were repeated by replacing different concentrations of nicotinic acid with lauric acid or kaempferol. Total cellular RNA of the cell samples were then extracted by using Tri Reagent ® LS (Molecular Research Center, USA) according to the manufacturer’s instruction.
Real Time Reverse Transcription Polymerase Chain Reaction (Real Time RT PCR)
One-step real-time RT-PCR was carried out by using the QuantiMix SYBR Green RT-PCR (Qiagen, Germany). The mixture with total volume of 20 μl was assembled on ice according to the following composition: 10 μl of QuantiMix SYBR Green RT-PCR, 6.6 μl of RNase-free water, 1 μl of forward primer (10 μM), 1 μl of reverse primer (10 μM), 0.4 μl Ribosafe RNase Inhibitor and 1 μl of RNA template. The primer sequences used are accordingly Matsuura et al. for ABCA1 [21] and Chew et al. (2007) for β-actin [22].
The samples were placed in Bio-Rad’s MyiQ™ Single- Color Real-Time PCR Detection System for quantitative PCR (qPCR), following a program, which consisted of 1 cycle each of 50 °C (for cDNA synthesis) for 10 min and 95 °C for another 3 min, followed by a 35 cycles of denaturing at 94 °C for 10 s, annealing at 60 °C for 30 s and primer extension at 72 °C for 30 s, before a melt curve analysis was carried out at the end of the qPCR cycle.
Normalization against housekeeping gene β-actin was performed in order to determine the relative mRNA expression of the target gene using delta-delta CT method and the calculated expression of ABCA1 mRNA was presented in fold-changes as compared to untreated control (fold-value equals to 1.00). Each experiment was performed in triplicates.
Statistical data analysis
Results were presented as means and standard deviations of triplicate determinations. Experimental groups were compared by using the SPSS Statistics Standard software, version 16.0 (IBM, USA). Oneway ANOVA was used for all analysis using the LSD algorithm at a 95% confidence interval. Differences were considered statistically significant at the p < 0.05 level.
Results
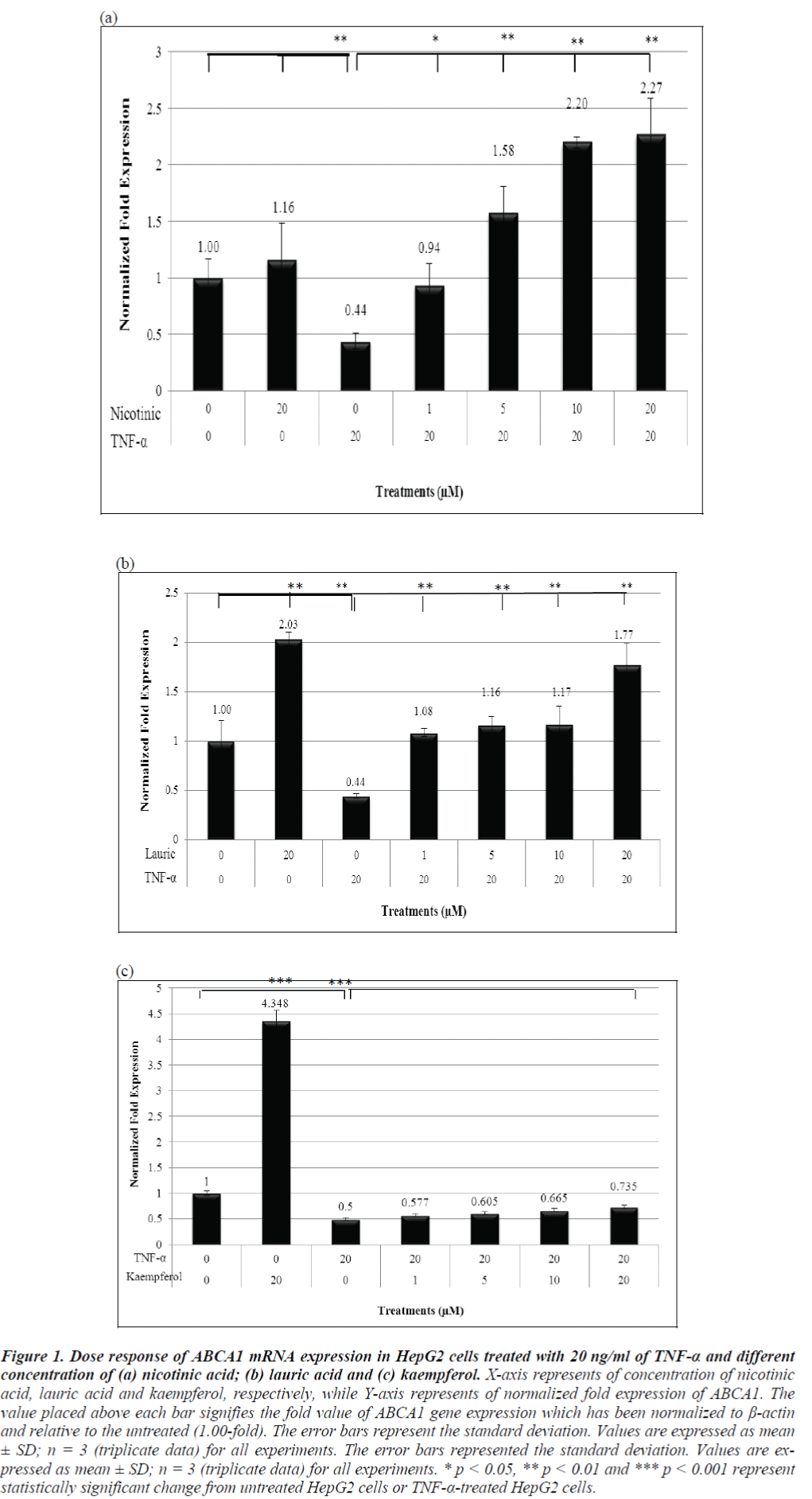
As shown in Figure 1a, the ABCA1 mRNA expression with only 20 μM nicotinic acid was up-regulated to 1.16-fold, as compared to the untreated sample but the increase was not significant. Interestingly, stimulation with 20 μM of lauric acid alone markedly increased ABCA1 mRNA expression in 2.03-fold. As expected, the expression of ABCA1 was significantly repressed in TNF-α-treated HepG2 cells to 0.44-fold. Subsequent treatment of TNF-α-pretreated HepG2 cells with 1 μM, 5 μM, 10 μM and 20 μM of nicotinic acid showed significant increase to 0.94-fold, 1.58-fold, 2.20-fold and 2.27-fold of ABCA1 mRNA expression, respectively.
Figure 1: Dose response of ABCA1 mRNA expression in HepG2 cells treated with 20 ng/ml of TNF-α and different concentration of (a) nicotinic acid; (b) lauric acid and (c) kaempferol. X-axis represents of concentration of nicotinic acid, lauric acid and kaempferol, respectively, while Y-axis represents of normalized fold expression of ABCA1. The value placed above each bar signifies the fold value of ABCA1 gene expression which has been normalized to β-actin and relative to the untreated (1.00-fold). The error bars represent the standard deviation. Values are expressed as mean ± SD; n = 3 (triplicate data) for all experiments. The error bars represented the standard deviation. Values are expressed as mean ± SD; n = 3 (triplicate data) for all experiments. * p < 0.05, ** p < 0.01 and *** p < 0.001 represent statistically significant change from untreated HepG2 cells or TNF-α-treated HepG2 cells.
Similarly, the HepG2 cells treated with increasing concentrations of lauric acid (1 μM, 5 μM, 10 μM and 20 μM) also showed significant up-regulation of the ABCA1 mRNA expression to 1.08-fold, 1.16-fold, 1.17-fold and 1.77-fold, respectively (Figure 1b). Kaempferol also gradually increased ABCA1 expression in a dose-dependent manner to 0.577-fold, 0.605- fold, 0.664-fold and 0.735-fold, with 1 μM, 5 μM, 10 μM and 20 μM of kaempferol, respectively (Figure 1c).
Kaempferol alone significantly induced ABCA1 expression to approximately 4.348-fold. Comparing the three antioxidants, all were able to alleviate the repression by TNF-α, but kaempferol had the weakest effect amongst the three.
Discussion
In the present study, all three antioxidants alleviated TNF- α –down-regulation of ABCA1 expression. TNF-α has been shown to be involved in the expression of ABCA1 mRNA gene [3,23]. The inflammatory stress triggered by TNF-α activates NF-κB, and this in turn inhibits PPARγ1, PPARα and liver X receptor-α (LXR-α) [20,24,25], and leads to the down-regulation of ABCA1 gene expression [3,23].
Nicotinic acid was shown to increase PPAR-γ transcriptional activity through the activation of PPAR-γ-LXR-α- ABCA1 pathway [9,26-28]. Meanwhile, the PPAR-α response element has been identified in LXR promoter, suggesting that PPAR-α is involved indirectly in the regulation of ABCA1 transcription activity through LXR pathway [29,30]. Therefore, up-regulation of PPAR-α would indirectly increase the ABCA1 expression and further elevate in HDL formation [31]. Nicotinic acid has been proven to increase apoA-I levels in HepG2 cells [32]. ApoA-I activates cellular cAMP signaling through ABCA1 transporter, which in turn stimulates ABCA1 phosphorylation and its expression [33].
Lauric acid could increase the LXR-α, which acts as a positive regulator for ABCA1 expression [34,35]. Lauric acid may up-regulate LXR, and this leads to the decrease in sterol regulatory element-binding protein (SREBP2), which binds to the E-box in the ABCA1 promoter. The inactivation of SREBP2 and stimulation of LXR have been shown to synergistically induce ABCA1 expression [36]. Aside from that, it is also hypothesized that lauric acid may activate PPAR-α nuclear receptor and PPAR-γ agonists, which further enhanced the ABCA1 mRNA expression in a PPAR-dependent manner through LXR activation [37]. However, more studies have to be carried out to confirm the actual mechanisms triggered by lauric acid.
Kaempferol is a natural product found abundantly in green tea, wine, vegetable and fruits. It has anti-oxidant and anti-inflammatory effects. Its effects were proven when ABCA1 mRNA expression pattern showed gradual increase in a given constant dose of TNF-α together with increased doses of kaempferol in this study. Similar scenario was also observed in our previous study with LXR-α [38]. Kaempferol has been shown to downregulate TNF-α production by interfering IκB kinase (IKK) and mitogen activated protein kinase (MAPK) to inhibit NF-κB in NF-κB pathway [39,40]. However, under the inflammatory state caused by the TNF-α stimulation, kaempferol failed to alleviate the ABCA1 expression back to untreated levels, although there was an alleviation in the expression levels. ABCA1 mRNA expression was up-regulated significantly with high dose of kaempferol alone. This shows a positive relationship between ABCA1 and kaempferol which suggests that kaempferol may be more suitable as a preventive substance which would benefit the host organism.
In summary, nicotinic acid, lauric acid and kaempferol have positive impacts on ABCA1 gene expression in the TNF-α stimulated HepG2 cells. The up-regulation of the gene is critical in maintaining cholesterol homeostasis and preventing cardiovascular disease. Thus, the induction in expression of the target genes may be a desirable antiatherogenic therapeutic approach.
Acknowledgement
The authors thank Dr. Ha Sie Tong for his contribution of lauric acid for the study. This work was supported by Department of Biomedical Science (UTAR) and some of the consumables used in this study were supported by UTARRF (Project Number IPSR/RMC/UTARRF/C2- 11/C04; Vote Number 6200/C45).
Conflict of interest statement
We declare that we have no conflict of interest.
References
- Leiva A, Verdejo H, Benítez ML, Martínez A, Busso D, Rigotti A. Mechanism regulating hepatic SR-B1 expression and their impact on HDL metabolism. Atherosclerosis 2011; 217(2): 299-307.
- Schmitz G, Langmann T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim Biophys Acta 2005; 1735(1): 1-19.
- Field FJ, Watt K, Mathur SN. TNF-α decreases ABCA1 expression and attenuates HDL cholesterol efflux in the human intestinal cell line Caco-2. J Lipid Res 2010; 51(6): 1407-1415.
- Liao H, Langmann T, Schmitz G, Zhu Y. Native LDL upregulation of ATP-binding cassette transporter 1 in human vascular endothelial cells. Arterioscler Thromb Vasc Biol 2002; 22(1): 127-132.
- Assmann G, von Eckardstein A, Brewer HB. Familial Analphalipoproteinemia: Tangier Disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw Hill 2001: 2937-2960.
- Choi S, Yoon H, Oh KS, Oh YT, Kim YI, Kang I, et al. Widespread effects of nicotinic acid on gene expression in insulin-sensitive tissues: implications for unwanted effects of nicotinic acid treatment. Metabolism 2011; 60(1): 134-144.
- Walldius G, Wahlberg G. Effects of nicotinic acid and its derivatives on lipid metabolism and other metabolic factors related to atherosclerosis. Adv Exp Med Biol 1985; 183: 281-293.
- Lakey WC, Greyshock N, Guyton JR. Adverse reactions of Achilles tendon xanthomas in three hypercholesterolemic patients after treatment intensification with niacin and bile acid sequestrants. J Clin Lipidol 2013; 7(2): 178-181.
- Zhang LH, Kamanna VS, Ganji SH, Xiong XM, Kashyap ML. Niacin increases HDL biogenesis by enhancing DR4-dependent transcription of ABCA1 and lipidation of apolipoprotein A-I in HepG2 cells. J Lipid Res 2012; 53(5): 941-950.
- Jin FY, Kamanna VS, Kashyap ML. Niacin accelerates intracellular ApoB degradation by inhibiting triacylglycerol synthesis in human hepatoblastoma (HepG2) cells. Arterioscler Thromb Vasc Biol 1999; 19(4): 1051-1059.
- Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redoxsensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis 2009; 202(1): 68-75.
- Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med 2005; 258(2): 94-114.
- Lieberman S, Enig MG, Preuss HG. A Review of Monolaurin and Lauric Acid. Alternative and Complementary Therapies 2006; 12(6): 310-314.
- Lottenberg AM, Afonso Mda S, Lavrador MS, Machado RM, Nakandakare ER. The role of dietary fatty acids in the pathology of metabolic syndrome. J Nutr Biochem 2012; 23(9): 1027-1040.
- Dimas K, Demetzos C, Mitaku S, Marselos M, Tzavaras T, Kokkinopoulos D. Cytotoxic activity of kaempferol glycosides against human leukaemic cell lines in vitro. Pharmacol Res 2000; 41(1): 83-86.
- Roth A, Schaffner W, Hertel C. Phytoestrogen kaempferol (3,40,5,7-tetrahydroxyflavone) protects PC12 and T47D cells from beta-amyloid-induced toxicity. J Neurosci Res 1999; 57(3): 399-404.
- Sathyamoorthy N, Wang TT, Phang JM. (1994). Stimulation of pS2 expression by diet-derived compounds. Cancer Res 1994; 54(4): 957-961.
- Wullaert A, Heyninck K, Beyaert R. Mechanisms of crosstalk between TNF-induced NF-κB and JNK activation in hepatocytes. Biochem Pharmacol 2006; 72(9): 1090-1101.
- van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist 2006; 11(4): 397-408.
- Lim WS, Ng DL, Kor SB, Wong HK, Tengku-Muhammad TS, Choo QC, et al. Tumour necrosis factor alpha down-regulates the expression of peroxisome proliferator activated receptor alpha (PPARα) in human hepatocarcinoma HepG2 cells by activation of NF-κB pathway. Cytokine 2013; 61(1): 266-274.
- Matsuura F, Oku H, Koseki M, Sandoval JC, Yuasa-Kawase M, Tsubakio-Yamamoto K, et al. Adiponectin accelerates reverse cholesterol transport by increasing high density lipoprotein assembly in the liver. Biochem Biophys Res Commun 2007; 358(4): 1091-1095.
- Chew CH, Chew GS, Najimudin N, Tengku-Muhammad TS. Interleukin-6 inhibits human peroxisome proliferator activated receptor alpha gene expression via CCAAT/enhancer-binding proteins in hepatocytes. Int J Biochem Cell Biol 2007; 39(10): 1975-1986.
- Gerbod-Giannone MC, Li Y, Holleboom A, Han S, Hsu LC, Tabas I, et al. TNF-alpha induces ABCA1 through NF-κB in macrophages and in phagocytes ingesting apoptotic cells. Proc Natl Acad Sci U S A 2006; 103(9): 3112-3117.
- Gao Z, He Q, Peng B, Chiao PJ, Ye J. Regulation of nuclear translocation of HDAC3 by IkappaBalpha is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor γ function. J Biol Chem 2006; 281(7): 4540-4547.
- Ma KL, Ruan XZ, Powis SH, Chen Y, Moorhead JF, Varghese Z. Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology 2008; 48(3): 770-781.
- Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med 2001 Jan; 7(1): 53-58.
- Wu ZH, Zhao SP. Niacin promotes cholesterol efflux through stimulation of the PPARgamma-LXRalpha-ABCA1 pathway in 3T3-L1 adipocytes. Pharmacology 2009; 84(5): 282-287.
- Di D, Wang Z, Liu Y, Luo G, Shi Y, Berggren-Söderlund M, et al. ABCA1 upregulating apolipoprotein M expression mediates via the RXR/LXR pathway in HepG2 cells. Biochem Biophys Res Commun 2012; 421(1): 152-156.
- Siripurkpong P, Na-Bangchang K. Effects of niacin and chromium on the expression of ATP-binding cassette transporter A1 and apolipoprotein A-1 genes in HepG2 cells. J Nutr Biochem 2009; 20(4): 261-268.
- Mogilenko DA, Shavva VS, Dizhe EB, Orlov SV, Perevozchikov AP. PPAR-γ activates ABCA1 gene transcription but reduces the level of ABCA1 protein in HepG2 cells. Biochem Biophys Res Commun 2010; 402(3): 477-482.
- Voloshyna I, Reiss AB. The ABC transporter in lipid flux and atherosclerosis. Prog Lipid Res 2011; 50(3): 213-224.
- Haas MJ, Alamir AR, Sultan S, Chehade JM, Wong NC, Mooradian AD. Nicotinic Acid induces apolipoprotein A-I gene expression in HepG2 and Caco-2 cell lines. Metabolism 2011; 60(12): 1790-1796.
- Haidar B, Denis M, Marcil M, Krimbou L, Genest J Jr. Apolipoprotein A-I activates cellular cAMP signalling through the ABCA1 transporter. J Biol Chem 2004; 279(11): 9963-9969.
- Ozasa H, Ayaori M, Iizuka M, Terao Y, Uto-Kondo H, Yakushiji E, et al. Pioglitazone enhances cholesterol efflux from macrophages by increasing ABCA1/ABCG1 expressions via PPARγ/LXRα pathway: findings from in vitro and ex vivo studies. Atherosclerosis 201; 219(1):141-50.
- Jun HJ, Hoang MH, Yeo SK, Jia Y, Lee SJ. Induction of ABCA1 and ABCG1 expression by the liver X receptor modulator cineole in macrophages. Bioorg Med Chem Lett 2013; 23(2): 579-83.
- Zhou X, Yin Z, Guo X, Hajjar DP, Han J. Inhibition of ERK1/2 and activation of liver X receptor synergistically induce macrophage ABCA1 expression and cholesterol efflux. J Biol Chem 2010; 285(9): 6316-6326.
- Ogata M, Tsujita M, Hossain MA, Akita N, Gonzalez FJ, Staels B, et al. On the mechanism for PPAR agonists to enhance ABCA1 gene expression. Atherosclerosis 2009; 205(2): 413-419.
- Yu YE, Chew CH. Downregulation in the mRNA expression of nuclear hormone receptor liver-Xreceptor alpha (LXR-α) by TNF-α is abolished by the antioxidant kaempferol, but not ascorbic acid, in human hepatocarcinoma HepG2 cells. Asian Biomedicine 2012; 6(4); 585 – 589.
- García-Mediavilla V, Crespo I, Collado PS, Esteller A, Sánchez-Campos S, Tuñón MJ, et al. (2007). The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and downregulation of the NF-κB pathway in Chang Liver cells. Eur J Pharmacol 2007; 557(2-3): 221-229.
- Kim HK, Park HR, Lee JS, Chung TS, Chung HY, Chung J. Down-regulation of iNOS and TNF-α expression by kaempferol via NF-κB inactivation in aged rat gingival tissues. Biogerontology 2007; 8(4): 399-408.