Research Article - Current Pediatric Research (2022) Volume 26, Issue 12
Myopericardial syndromes in a pediatric cohort: A 5-year observational study.
Marina Pons-Espinal1*, Sara Bobillo-Pérez2,3, Silvia Simó-Nebot4, Mònica Girona-Alarcon2,3, Mònica Balaguer Gargallo2,3, Joan Sanchez-de-Toledo5,6, Iolanda Jordan-García2,7,8
1Department of Pediatrics, Hospital Sant Joan de Déu, University of Barcelona, Barcelona, Spain
2Pediatric Intensive Care Unit, Hospital Sant Joan de Déu, University of Barcelona, Barcelona, Spain
3Department of Immunological and Respiratory Disorders, Pediatric Critical Patient Research Group, Institut de Recerca Sant Joan de Déu, Hospital Sant Joan de Déu, Barcelona, Spain
4Department of Pediatrics, Infectious and Imported Diseases Unit, Hospital Sant Joan de Déu, University of Barcelona, Barcelona, Spain
5Department of Pediatric Cardiology, Hospital Sant Joan de Déu, University of Barcelona, Barcelona, Spain
6Department of Critical Care Medicine, Children’s Hospital of Pittsburgh, University of Pittsburgh, PA, US
7Pediatric Infectious Diseases Research Group, Institut de Recerca Sant Joan de Deu, Barcelona, Spain
88CIBER en Epidemiología Salud Pública (CIBERESP), Madrid, Spain
- *Corresponding Author:
- Marina Pons-Espinal
Department of Pediatric
Hospital Sant Joan de Déu
University of Barcelona
Barcelona, Spain
E-mail: mponse@hsjdbcn.es
Received: 25 November, 2022, Manuscript No. AAJCP-22-83656; Editor assigned: 28 November, 2022, Pre QC No. AAJCP-22-83656(PQ); Reviewed: 05 December, 2022, QC No. AAJCP-22-83656; Revised: 23 December, 2022, Manuscript No. AAJCP-22-83656(R); Published: 30 December, 2022, DOI:10.35841/0971-9032.26.12.1711-1718.
Abstract
Introduction: There is a clinical spectrum of myopericardial syndrome regarding the involvement of the myocardium, from myopericarditis to myocarditis.
Objectives: The aim of this study was to describe and compare the clinical presentation, the therapeutic management and the clinical evolution of myocarditis and myopericarditis.
Methods: This was a retrospective observational study in a third level pediatric hospital, including children <18 years old diagnosed with myocarditis or myopericarditis from June 2015 to June 2020.
Results: There were included 24 patients (15 myocarditis and 9 myopericarditis). Only myocarditis presents with respiratory symptoms and abnormal chest radiograph, as well as worse general condition in the emergency department (p<0.01). Patients with myocarditis were younger, 14.4 months (IQR=3-60) vs. 197.5 (IQR=183-208) (p<0.01), presented with more tachycardia (p<0.01) and more metabolic acidosis (p=<0.01). On echocardiography, they had worst ventricular ejection fraction, 35% (IQR=25-40) vs. 71% (IQR=60-75), p<0.01. Fourteen myocarditis (93%) were admitted to the intensive care unit; five (36%) were placed on extracorporeal membrane oxygenation. Patients with myocarditis had significantly longer admissions, 23 days (IQR=12-38) vs. 5 (IQR=2-8) (p<0.01).
Conclusion: Myocarditis had a more severe clinical presentation, and required more frequently intensive care. Respiratory symptoms may help to predict poor prognosis in these patients.
Keywords
Myocarditis, Myopericarditis, Intensive care.
Introduction
Acute myocarditis is a severe inflammatory heart disease that has a broad clinical expression [1,2]. The degree of involvement of the pericardium and the myocardium determines both, the clinical presentation and its severity and it has also been used to classify the myopericardial syndromes. A wide spectrum of clinical expression exists ranging from pure pericardial involvement (pericarditis) to increasing degrees of myocardial involvement including myopericarditis, perimyocarditis and pure myocarditis [3,4]. Although myopericarditis and perimyocrditis are often used indistinctively, myopericarditis refers to a pericardial syndrome with myocardial involvement and perimyocarditis describes a primarily myocardial syndrome with pericardial involvement. Acute myocarditis is a serious disease with an estimated incidence of 1 case per 100,000 children [5].
Consensus on the diagnostic criteria as well as on the therapeutic approach or follow-up of pediatric patients has yet to be reached [6]. The time required for diagnosis, the correct patient referral and a prompt treatment are of paramount importance to ensure recovery and mitigate risk of clinical deterioration [7]. Several studies have attempted to define the clinical, analytical, and echocardiographic signs associated with higher mortality, such as hypotension, dyspnea, low ejection fraction, high troponins and high brain natriuretic peptide or N-terminal-pro-hormone brain natriuretic peptide [8-11]. Cardiac Magnetic Resonance Imaging (cMRI) represents the noninvasive gold standard test for the diagnosis of acute myocarditis [6,12].
The endomyocardial biopsy is used only in certain cases despite being the gold standard test due to the associated risk [6,13]. This study aimed to describe and compare the clinical and analytical characteristics of myocarditis and myopericarditis. The secondary objective was to analyze the risk factors of poor prognosis in patients with myopericardial syndrome.
Materials and Methods
This was a retrospective observational single-center study from 2015 to 2020 performed in a referral tertiary pediatric hospital. The inclusion criteria were children <18 years old with a probable or confirmed diagnosis of acute myocarditis or myopericarditis. Exclusion criteria: dilated cardiomyopathy or heart failure with any etiology different from myocarditis; underlying congenital heart disease, suspicion of inborn error of metabolism, neuromuscular disorders, Kawasaki disease, and immunosuppressed patients.
The definition of myocarditis was based on the diagnostic criteria proposed by Sagar, et al. [14]. Probable acute myocarditis was considered in presence of cardiovascular symptoms and at least one of the following: (1) Raised in cardiac injury biomarkers; (2) Suggestive electrocardiogram findings of cardiac injury; (3) Abnormal cardiac function on echocardiogram or cMRI. The confirmatory diagnosis of acute myocarditis was obtained with the presence of compatible histological findings, through an endomyocardial biopsy [14,15]. Left ventricular systolic dysfunction was defined as left ventricular ejection fraction <50%. Dilation of the left ventricle was defined as its diastolic diameter >2 Z-score for the body surface area.
According to the guidelines for the diagnosis and management of pericardial disease by the European cardiology society in 2015, the definition and diagnosis of myopericarditis can be clinically established if patients with definite criteria for acute pericarditis show elevated biomarkers of myocardial injury without newly developed focal or diffuse impairment of left ventricular function in echocardiography or cMRI. Perimyocarditis should be diagnosed in patients with clinical criteria for acute pericarditis, the elevation of cardiac markers of injury, and evidence of the new onset of focal or diffuse depressed left ventricular function by echocardiography or cMRI. The eventual diagnosis was based on the clinical impression of the attending cardiologist [16].
Variables
Medical records were reviewed including data from the emergency department to one-year follow-up after discharge. Data included were demographics, clinical data in the emergency department, laboratory findings, microbiological data (serology and polymerase-chain-reaction, complementary tests results (electrocardiogram, echocardiogram, biomarkers, chest x-ray), management and prognostic data. Supportive management recorded included the hemodynamic support, analyzed as the need for extracorporeal membrane oxygenation, need for inotropic and the maxim vasoactive-inotropic score [17], and the respiratory support, analyzed as the need for mechanical ventilation and its duration. Likewise, the length of stay in the Pediatric Intensive Care Unit (PICU) and hospital was recorded, as well as in-hospital mortality. Poor prognosis was defined as the appearance of left ventricular systolic dysfunction (EF<30%) or dilation at hospital discharge, need for a heart transplant or death [10].
Statistical analysis
Categorical variables expressed as frequency and percentages and compared using the chi-square test or the fisher's exact test; continuous variables expressed as the median and Interquartile Range (IQR) and compared using the Mann- Whitney test. The multivariate logistic regression analysis was performed of those variables selected in the univariate analysis to determine if there are independent factors of poor prognosis in myocarditis. These results were expressed as Odds Ratio (OR). To examine the accuracy of biomarkers to predict the need for extracorporeal membrane oxygenation, a Receiver- Operating Characteristic (ROC) was plotted and the Area Under the Curve (AUC) quantified. p<0.05 was considered significant SPSS.25.0®
Institutional review board statement: The study was conducted according to the guidelines of the declaration of Helsinki, and approved by the institutional review board of CEIm Fundació Sant Joan de Déu (PIC-214-19, on the 28th November 2019).
Informed consent statement: The local ethical assistance committee and the institutional review board waived the parental informed consent due to the retrospective and descriptive design.
Results
Clinical and analytical features
Twenty-four patients were included, sixteen (67%) were males and the median age was 72 months (9-195). Three patients (12%) were newborns. According to the diagnosis, patients were classified into myocarditis (n=15), and myopericarditis (n=9). None of the patients responded to the definition of perimyocarditis. The most common presentations in the emergency department were fever (n=18, 75%) and gastrointestinal symptoms (n=14, 58%: vomits (n=8), poor feeding (n=3), diarrhea (n=2) and abdominal pain (n=1)). Twelve patients (50%) presented respiratory symptoms, 9 (58%) chest pain, and 3 (12) had neurological symptoms. Twelve patients (50%) had a bad general condition, meaning two abnormal sides of the pediatric assessment triangle in the emergency department. Table 1 compares patients with myocarditis vs. patients with myopericarditis.
| Myocarditis (n=15) | Myopericarditis (n =9) | p-value | |
|---|---|---|---|
| Demographic data | |||
| Age(months) | 14.4(3.3-59.7) | 197.5(183.3-208.5) | <0.001* |
| Male(%) | 8(53) | 8(89) | 0.17 |
| Underlyingpathology (%) | 4(26) | 1(11) | 0.61 |
| Clinicaldata | |||
| Badgeneral condition (%) | 8(53) | 0 (0) | 0.009* |
| Timeof evolution (hours) | 72(24-96) | 24(12-66) | 0.08 |
| Fever(%) | 9(60) | 9(100) | 0.05 |
| Maximumtemperature (ºC) | 38.5(38.3-39.1) | 38.6(37.6-39.2) | 0.75 |
| Durationof fever (hours) | 60(5-78) | 48(24-57) | 0.83 |
| Tachycardia(%) | 13(87) | 4(44) | 0.06 |
| Tachycardia(percentile) | 99(96-99) | 92.5(90-95) | 0.006* |
| Tachypnea(%) | 11(73.3) | 1(11.1) | 0.009* |
| Tachypnea(percentile) | 95(80-97) | - | - |
| Dyspnea(%) | 12(80) | 0 (0) | <0.001* |
| Thoracicpain (%) | 0 (0) | 9(100) | <0.001* |
| Gastrointestinalsymptoms (%) | 10(67) | 4 (44) | 0.4 |
| Neurologicalsymptoms (%) | 2(13) | 1(11) | 1 |
| Arrhythmias(%) | 2(13) | 0 (0) | 0.51 |
| Complementarytests | |||
| CRP(mg/L) | 7.05(2.47-21.05) | 53(30.7-129.9) | 0.002* |
| PCT(ng/mL) | 0.35(0.13-1.14) | 0.14(0.09-0.16) | 0.32 |
| Lactate(mmol/l) | 2.2(1.3-3.2) | 1.3 (1.1-1.9) | 0.14 |
| pH | 7.3(7.23-7.39) | 7.38(7.36-7.40) | 0.05 |
| Baseexcess (mmol/L) | -4.9 (-11-(-1.4)) | -0.5(-1.6-(-0.25) ) | 0.002* |
| TroponinI (ng/ml) | 0.69(0.1-7.9) | 7.24(4.9-25) | 0.013* |
| NT-proBNP(ng/L) | 16477(7224-31429) | 403(216-2079) | 0.07 |
| AST(UI/L) | 27 (14-71) | 24(17.5-47.5) | 0.78 |
| ALT(UI/L) | 84.5(28-328) | 59(45-107) | 1 |
| AbnormalECG (%) | 7(47) | 7(78) | 0.21 |
| Abnormalchest x-ray (%) | 13(86.7) | 0 (0) | 0.009* |
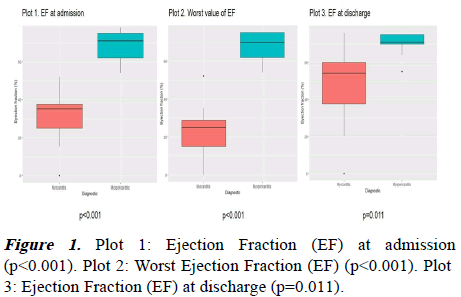
| Ejectionfraction | 35(25-40) | 71(60-75) | <0.001* |
| Evolution | |||
| HospitalLOS | 23(12-38) | 5(2-7.5) | <0.001* |
| Mortality,n (%) | 1 (7) | 0 (0) | 1 |
Note: *Categoricalvariables expressed by frequency (percentage) and compared using the chi-squaretest. Continuous variables expressed by median (interquartile range) andcompared using the Mann-Whitney test.
Abbreviations: CRP: C-Reactive Protein; PCT: Procalcitonin; NT-proBNP: N-TerminalPro-Brain Natriuretic Peptide; AST: Aspartate Aminotransferase; ALT: AlanineAminotransferase; ECG: Electro Cardiogram. Time of evolution (hours) meantsymptoms to ER presentation.
Table 1. The comparison between myocarditis and myopericarditis presentation.
Complementary tests
Regarding the complementary tests, chest x-rays were only altered in children with myocarditis: 13 children (87%) had different findings in the chest x-ray, being the acute pulmonary edema the most common finding (53%), followed by pulmonary infiltrates (n=4, 27%) and cardiomegaly (n=4, 27%). The ejection fraction at admission decreased in patients with myocarditis (35% vs. 71%, p<0.001). The most common findings were sinus tachycardia and unspecific ST-T wave changes. As shown in Table 1, patients with myocarditis had worse metabolic acidosis, but lower C-reactive protein and troponin-I values compared to patients with myopericarditis. No differences were observed with respect the lactate.
Nine patients (37%) presented arrhythmias during the admission, mainly observed in patients with myocarditis (53% vs. 11%, p=0.08). Figure 1 represents the different ejection fraction values for myocarditis and myopericarditis. The presence of pericardial effusion was similar in both groups (p=1.00).
cMRI was performed in 7 patients (3 myocarditis, 21% and 4 myopericarditis, 44%), and only one was performed during the admission. The median time until the cMRI was 5 months (IQR 2-16). Three had pathological findings; two cMRI were performed during the admission and showed sever ventricular dilation. The third one (the only one with a diagnosis of myopericarditis) was performed sixteen months after admission only showed late gadolinium enhancement, that was also seen in one of the patients with myocarditis.
A microbiological diagnosis was achieved in 14 patients (58%) with a positive polymerase-chain-reaction, and just one was a myopericarditis. The most common finding was parvovirus- B19, detected in 6 cases (25%), and followed by enterovirus (20%). Enterovirus caused all the neonatal myocarditis. An EMB was performed in only three cases of myocarditis (20%).
Immunoglobulin treatment was used in 78% of patients: in the 93% of myocarditis and in the 56% of myopericarditis (p=0.056). Myocarditis received higher dose of immunoglobulin: 3 g/kg (IQR 2-3) vs. 1 (IQR=0.2-2.8), p=0.02. Steroids were only used in myocarditis (50%), p=0.019. In two cases of parvovirus-B19 myocarditis, the interferon-beta was used as a late therapeutic option due to a chronic viral infection. The 69.6% of the patients received antibiotics: 12 myocarditis, 86%, and 4 myopericarditis, 44%, p=0.066. Macrolides were widely used: 57% myocarditis and 44% myopericarditis (p=0.68). The duration of the antibiotics trended to be longer in the myocarditis group: 7 days (IQR=3-10) vs. 3 (IQR=0-7.8), p=0.180.
Intensive support
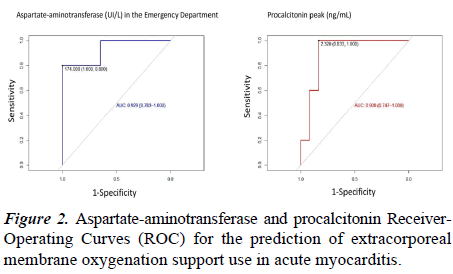
Sixteen patients (67%) were admitted to the PICU: 14 myocarditis (93%), and 2 myopericarditis (22%). One myocarditis died in the emergency department after an irreversible cardiac arrest. Patients with myopericarditis did not require intensive support, and they stayed 1 and 3 days for hemodynamic monitoring. In contrast, the need for intensive support was frequent in myocarditis: 10 patients (71%) required mechanical ventilation during a median of 12 days (IQR=6.5-21), and 13 (92%) needed vasoactive support, with a maxim vasoactive inotropic score of 17 (IQR=9.1-25.2). Nine (64%) required levosimendan. Five patients (36%) required extracorporeal membrane oxygenation due to cardiogenic shock: 80% due to severe ventricular dysfunction and 20% due to intractable arrhythmia. PICU length of stay for myocarditis was 13 days (IQR=5.8-21.3). Patients who required extracorporeal membrane oxygenation had higher values of aspartate-aminotransferase in the emergency department, higher procalcitonin values and required longer PICU and hospital length of stay (Table 2). The AUC for predicting need for extracorporeal membrane oxygenation in myocarditis was greater for aspartate aminotransferase than for procalcitonin (Figure 2).
| No ECMO support (n=9) | ECMO support (n=5) | p | |
|---|---|---|---|
| Demographicdata | |||
| Age(months) | 17.6(6-92) | 7.5(0.3-37.75) | 0.13 |
| Male(%) | 5(56) | 2(40) | 1 |
| Newborn(%) | 1(11.1) | 2(40) | 0.5 |
| Clinicaldata | |||
| Timeof evolution (hours) | 72(30-132) | 48(18-72) | 0.24 |
| Feverat admission (%) | 5(56) | 3(60) | 1 |
| Durationof fever (hours) | 72(60-132) | 4.5(1.5-55.5) | 0.06 |
| Badgeneral condition at ED (%) | 3(33.3) | 5(100) | 0.031* |
| Gastrointestinalsymptoms (%) | 6(67) | 3(60) | 1 |
| Respiratorysymptoms (%) | 8(89) | 4(80) | 1 |
| Tachycardiaat admission (%) | 7(78) | 5(100) | 0.5 |
| Tachypneaat admission (%) | 7(78) | 4(80) | 1 |
| Complementarytests | |||
| AbnormalECG at admission (%) | 3(33) | 4(80) | 0.27 |
| CRPat admission (mg/L) | 6.6(1.32-16.52) | 7.1(2.4-20.5) | 0.73 |
| CRRmaximum value (mg/L) | 18.8(3.9-110.20) | 24.8(4.3-43.35) | 1 |
| PCTat admission (ng/mL) | 0.14(0.06-0.72) | 0.98(0.39-2.26) | 0.045* |
| PCTmaximum value (ng/mL) | 0.54(0.11-3.9) | 4.14(2.6-14.98) | 0.07 |
| pH atadmission | 7.3(7.26-7.39) | 7.23(7.05-7.31) | 0.19 |
| Baseexcess at admission (mmol/L) | -4.9(-2–(-9.45)) | -5.5(-0.65-(-18)) | 1 |
| Lactateat admission (mmol/L) | 1.4(1.17-3.35) | 2.5(1.7-2.9) | 0.61 |
| TroponinI peak (ng/ml) | 1.05(0.35-3.80) | 13.2(6.35-28.36) | 0.11 |
| NT-pro-BNPpeak (ng/L) | 16477(12416-16907) | 36611(9857-160752) | 0.29 |
| AST(UI/L) | 33(14.25-77.75) | 209(128-1191) | 0.006* |
| AST>175UI/L (%) | 1(11) | 4 (80) | 0.023* |
| ALT(UI/L) | 25(14-30.50) | 86(14.5-527) | 0.29 |
| LVejection fraction at admission (%) | 35(25-37) | 30(22-40) | 0.79 |
| WorstLV ejection fraction (%) | 28(22-35) | 15(15-20) | 0.012* |
| Therapeuticsupport | |||
| MaximVIS score (points) | 13.2(5.6-19.3) | 23.7 (16.9-48.4) | 0.09 |
| IVIG(%) | 8(89) | 5(100) | 1 |
| Steroids(%) | 3(33) | 4(80) | 0.26 |
| Evolution | |||
| PICULOS (days) | 10(4-17) | 20(15.5-34) | 0.029* |
| HospitalLOS (days) | 16(11.5-34.5) | 27(24-46.5) | 0.14 |
Note: *Categorical variablesexpressed by frequency (percentage) and compared using the Chi- square test.Continuous variables expressed by median (interquartile range) and comparedusing the Mann-Whitney test.
Abbreviations: ECMO: Extra Corporeal Membrane Oxygenation; IQR: Interquartile Range;ED: Emergency Department; ECG: Electrocardiogram; CRP: C-Reactive Protein;PCT: Procalcitonin; NT-proBNP: N-Terminal Pro-Brain Natriuretic Peptide; AST:Aspartate Aminotransferase; ALT: Alanine Aminotransferase; LV: Left Ventricle;VIS: Vasoactive Inotropic Score; IVIG: Intravenous Immunoglobulin; PICU:Pediatric Intensive Care Unit; LOS: Length of Stay.
Table 2. Comparison between the ECMO vs. the non-ECMO group in myocarditis.
Outcomes
All patients with myopericarditis had favorable hospital course and were discharged without any sequelae. Patients with myocarditis had significantly longer hospital length of stay. Half of the myocarditis presented a poor prognosis at discharge (n=8, 53%). Seven patients (47%) presented cardiac dilation at hospital discharge and one patient died. After one year, 12 (80%) had a normal ejection fraction, one patient evolved to a dilated cardiomyopathy and one patient required a cardiac transplant two months after discharge.
In the multivariate analysis, only respiratory symptoms in the emergency department were independently associated with a poor prognosis: OR 15.4, p=0.022. Hospital length of stay was longer in patients with poor prognosis (17 days (IQR=14-53) vs. 8 (2.8-24.5), p=0.027).
Discussion
This study analyses pediatric patients with myopericardial syndrome. As it is already known, it is observed that children with myocarditis required significantly longer admissions and had worst outcomes at discharge compared to patients with myopericarditis. Younger age, presence of respiratory symptoms, tachypnea and tachycardia at the initial emergency department assessment were more frequent in patients with myocarditis, whereas all patients with myopericarditis presented with chest pain and fever but respiratory symptoms were absent. As it has been previously described by Freedman et al. respiratory symptoms in patients with myocarditis were the most common finding [7].
The risk for poor prognosis associated with respiratory symptoms was statistically significant, with OR 15.4 (IQR=1.5-161). A worst left ventricular ejection fraction at admission and its lower value, and also a longer evolution of symptoms (>72 hours) were associated with worst outcome, consistently with previous studies [10], although it was not statistically significant in the univariate analysis. Other risk factors as age or tachycardia were not significantly associated with a worst prognosis, although they have previously been described [10].
Surprisingly, patients with myocarditis had lower values of troponins at admission, and achieved lower peaks in our sample, while in previous studies troponin levels were higher in severe myocardium affection [18,19]. It has to be taken into account that troponin-I may result from reversible membrane damage and not necessarily from cell death [20] and also, the different age between two groups in our sample. The median age of patients with myocarditis is 14.4 months so they are not able to fully communicate that they are feeling ill. This may lead to delay in diagnosis of myocarditis and could explain the lower troponin levels in this group compared to the myopericarditis group. The troponin-I levels could have been higher but had fallen by the time the diagnosis was made. It is confirmed that troponin-I is helpful for the diagnosis of myopericarditis although does not permit to distinguish between myocarditis and myopericarditis.
Although a predominant involvement of the pericardial membrane in patients with myopericarditis was expected, there were no statistically significant differences regarding the pericardial effusion. No pericardial rubs were identified and the electrocardiogram changes were unspecific in both cases. However, it has to be considered that in fulminant myocarditis, it is common to find pericardial effusion, due to generalized edema in the myocardium [21]. The three patients with myocarditis and pericardial effusion responded to the definition of fulminant myocarditis.
Our results show that a microbiological diagnosis was achieved in up to 60%. This was more likely in myocarditis, and has to be interpreted assuming the severity of the pathology, that implied longer admissions and the performance of an exhaustive microbiological study. Enterovirus and parvovirus- B19 have been well described as possible trigger infections in myocarditis. In our study, three cases of rhinovirus enterovirus were identified by a mixed-polymerase-chain-reaction detection kit, which did not allow differentiating between the two viruses. There is a lack of evidence regarding the pathogenic role of the rhinovirus in myocarditis [22] but the possibility cannot be dismissed. In all cases, the diagnostic method to identify this pathogen was polymerase-chainreaction. The serology, performed in 83% of the cases, showed negative results.
As previous studies stated, this fact highlights the lack of evidence to support the determination of viral serology in myopericardial syndromes, taking into account the good results obtained using polymerase-chain-reaction in comparison with the serology [23]. In addition, an endomyocardial biopsy can be decisive in identifying infectious etiology in cases of unknown causative agent. Although direct viral damage exists, the main myocardial inflammation seems to be secondary to the immunologic response unleashed by the virus [24]. Currently, different therapeutic strategies in myocarditis are controversial, although widely extended as our results show. Almost 90% of myocarditis received immunoglobulin’s in our study, but differences were not observed regarding the prognosis. An analysis of the pediatric cardiomyopathy registry did not show an association between immunoglobulin’s with steroids and higher survival rate or improvement of the ventricular function [25], and a recent meta-analysis concluded that the systematic use of immunoglobulin’s cannot be recommended considering the current evidence [24].
Interferon-beta is beneficial in chronic viral infections, increasing the viral clearance in some cases (i.e. adenovirus or enterovirus), and inhibiting the viral reactivation (i.e. parvovirus-B19) [26]. In adults with refractory chronic cardiac failure and persistence of adenovirus or enterovirus in the endomyocardial biopsy, interferon-beta has demonstrated effectivity [26,27]. In our study, two patients with identified parvovirus-B19 were treated with Interferon-beta and in both cases were used in a subacute/chronic phase. Regarding other therapeutic options, a wide spectrum of antibiotics was more frequently used in myocarditis than myopericarditis. An acute severe condition may justify this approach. Patients with myocarditis that required extracorporeal membrane oxygenation represented the 36%; This is a high proportion comparing with previous study in which only the 7% of myocarditis required this support., although that could be explained regarding the young age of our sample [28]. The factors associated with the early prediction for extracorporeal membrane oxygenation support in children with myocarditis are still unclear. Aspartate aminotransferase elevation was described as common findings in myocarditis. Our results have shown that high levels of aspartate aminotransferase in the emergency department might predict the need for extracorporeal membrane oxygenation, as Wu et al. suggested [29].
Conclusion
Although elevations of aspartate aminotransferase are not specific for myocarditis, they might translate a severe organ dysfunction secondary to the cardiogenic shock and in our patients a relationship between high levels of aspartate aminotransferase and need for extracorporeal membrane oxygenation was clearly observed. Hence, this hepatic enzyme could be relevant to identify those patients with a more severe affectation who may require promptly mechanical support. A worst ejection fraction was also a risk factor to be placed on extracorporeal membrane oxygenation, as other studies show.
Acknowledgment
I would like to thank the intensive care unit department, the infectious disease unit and the cardiology department for their support.
References
- Levine MC, Klugman D, Teach SJ. Update on myocarditis in children. Curr Opin Pediatr 2010; 22: 278–83.
- Kühl U, Schultheiss HP. Myocarditis in children. Heart Fail Clin 2010; 6: 483–96.
- Imazio M, Trinchero R. The spectrum of inflammatory myopericardial diseases. Int J Cardiol 2010; 144(1): 134;
- Imazio M, Trinchero R. Myopericarditis: Etiology, management, and prognosis. Int J Cardiol 2008, 127, 17–26;
- Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med 2003; 348: 1647–55.
- Dasgupta S, Iannucci G, Mao C, et al. Myocarditis in the pediatric population: A review. Congenit Heart Dis. 2019, 14, 868–77;
- Freedman SB, Haladyn JK, Floh A, et al. Pediatric myocarditis: Emergency department clinical findings and diagnostic evaluation. Pediatrics 2007; 120(6): 1278–85.
- Abrar S, Ansari MJ, Mittal M, et al. Predictors of mortality in paediatric myocarditis. J Clin Diagn Res 2016; 10(6): SC12-SC16.
- Sachdeva S, Song X, Dham N, et al. Analysis of clinical parameters and cardiac magnetic resonance imaging as predictors of outcome in pediatric myocarditis. Am J Cardiol 2015; 115(4): 499–504:
- Rodriguez-Gonzalez M, Sanchez-Codez MI, Lubian-Gutierrez M, et al. Clinical presentation and early predictors for poor outcomes in pediatric myocarditis: A retrospective study. World J Clin Cases 2019; 7(5): 548–61.
- Chang YJ, Hsiao HJ, Hsia SH, et al. Analysis of clinical parameters and echocardiography as predictors of fatal pediatric myocarditis. PLoS One 2019; 14(3): e0214087.
- Banka P, Robinson JD, Uppu SC, et al. Cardiovascular magnetic resonance techniques and findings in children with myocarditis: A multicenter retrospective study. J Cardiovasc Magn Reson 2015; 17: 96.
- Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American heart association, the American college of cardiology, and the European society of cardiology endorsed by the heart failure society of association of the European society of cardiology. J Am Coll Cardiol 2007; 50: 1914–31.
- Sagar S, Liu PP, Cooper LT. Myocarditis. Lancet 2012; 379: 738–47.
- Caforio ALP, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J 2013; 34(33): 2636–48.
- Adler Y, Charron P, Imazio M, et al. ESC Guidelines for the diagnosis and management of pericardial diseases. Eur Heart J 2015; 36(42): 2921–64.
- Davidson J, Tong S, Hancock H, et al. Prospective validation of the vasoactive-inotropic score and correlation to short term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Med 2012; 38: 1184–90.
- Yoldaş T, Örün UA. What is the significance of elevated troponin-I in children and adolescents? A diagnostic approach. Pediatr Cardiol 2019; 40(8): 1638–44.
- Smith SC, Ladenson JH, Mason JW, et al. Elevations of cardiac troponin-I associated with myocarditis. Circulation 1997; 95(1): 163–8.
- Eerola A, Poutanen T, Savukoski T, et al. Cardiac troponin-I, cardiac troponin-specific autoantibodies and natriuretic peptides in children with hypoplastic left heart syndrome. Interact Cardiovasc Thorac Surg 2014; 18(1): 80–85.
- Kociol RD, Cooper LT, Fang JC, et al. Recognition and initial management of fulminant myocarditis: A scientific statement from the American heart association. Circulation 2020; 141(6): e69–e92;
- Chow J, Murphy J, Subedi A, et al. Rhinovirus-associated dilated cardiomyopathy. ID Cases 2020; 19: e00702.
- Mahfoud F, Gärtner B, Kindermann M, et al. Virus serology in patients with suspected myocarditis: Utility or futility? Eur Heart J 2011; 32: 897–903.
- Yen CY, Hung MC, Wong YC, et al. Role of intravenous immunoglobulin therapy in the survival rate of pediatric patients with acute myocarditis: A systematic review and meta-analysis. Sci Rep 2019; 9: 10459.
- Foerster SR, Canter CE, Cinar A, et al. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood: An outcomes study from the pediatric cardiomyopathy registry. Circ Hear Fail 2010; 3: 689–97.
- Crişan S, Tint D, Petrescu L. Therapeutic advances in emergency cardiology. Am J Ther 2019; 26(2): e294–e300;
- Kühl U, Pauschinger M, Schwimmbeck PL, et al. Interferon-β treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation 2003; 107: 2793–8.
- Klugman D, Berger JT, Sable CA, et al. Pediatric patients hospitalized with myocarditis: A multi-institutional analysis. Pediatr Cardiol 2010; 31: 222–8.
- Wu H-P, Lin M-J, Yang W-C, et al. Predictors of extracorporeal membrane oxygenation support for children with acute myocarditis. Biomed Res Int 2017; 2017: 2510695.