Research Article - Biomedical Research (2017) Volume 28, Issue 12
Morphometry of Rabbit anatomical regions used as experimental models in implantology and oral surgery
Eduardo Borie1*, Ricardo Calzzani2, Fernando José Dias1, Ramon Fuentes1 and Carlos Salamanca3
1Research Centre in Dental Sciences (CICO), Dental School, Universidad de La Frontera, Temuco, Chile
2Ribeirao Preto Dental School, University of São Paulo, Ribeirao Preto, SP, Brazil
3Universidad Adventista de Chile, Chillan, Chile
- *Corresponding Author:
- Eduardo Borie
Research Centre in Dental Sciences
Dental School
Universidad de La Frontera, Chile
Accepted date: April 28, 2017
Abstract
The animal model has been widely used in the biomaterials field. However, there are no morphometric patterns to create critical size defects in different areas used in implantology and oral surgery, based on the anatomy and histology of the rabbit’s tissue. Thus, the aim of this study is to provide measurements and limits for future research on the different anatomical regions used to evaluate biomaterials and/or surgical procedures in rabbits as animal models. Twenty-two rabbit heads were used in the study. The soft tissue was removed and the calvaria, body of the mandible divided into three regions, the ramus of the mandible, total mandible length and lower cortical sinus thickness were measured. The mean, standard deviation and confidence of each region were recorded. The height of the mandibular ramus was 30.1 mm and 29.7 mm for the right and left sides, respectively, while the total body of mandible was 67.2 mm. Also, the thickness of calvaria and lower cortical sinus were 1.5 mm and 1.1 mm, respectively. In conclusion, the limited dimensions of anatomical structures should be considered when working with critical size defects in rabbits to avoid complications or accidents during the specimen’s surgery.
Keywords
Rabbit, Morphometry, Anatomy, Implantology, Oral surgery
Introduction
The development of bioactive materials for tissue engineering has increased considerably for use on in vitro and in vivo models [1]. Accordingly, the animal model has been widely used in the study of biomaterials, placed in critical size defects, to analyse their influence [2]. The literature shows the use of dog [3,4], rat [5] and rabbit models [6], among others, to study bone healing.
Among the different animal models, one of the most frequently used is the rabbit due to low cost, easy manipulation, high bone metabolism and quick craniofacial development [7]. Research focused on bone healing in rabbits was conducted in different regions of the mandible [8] and calvaria [9]. However, the anatomy and size of each region of rabbit skull and mandible may influence the number of critical size defects.
The applied anatomy enables the clinician to identify details of relevant structures intraoperatively [10]. In this context, a study by Valdivia et al. [11] reported some measurements of rabbit mandible after transection of inferior alveolar nerves in growing rabbits. Despite the large amount of information available in the literature, there are no morphometric patterns to create critical size defects in different areas used in implantology and oral surgery based on the anatomy and histology of the rabbit’s tissue that would enable exact repeatability of methodologies and rendering more reliable results. Therefore, this study aims to provide measurements and limits for future research on the different anatomical regions used to evaluate biomaterials and/or surgical procedures using rabbits as animal models.
Material and Methods
The following study was approved by the Ethics Committee of the Faculty of Ribeirao Preto Dental School, University of São Paulo (Protocol 055/2012).
Twenty-two rabbit heads discarded from other research that were not included or have not caused metabolic bone diseases were used to conduct the study. The samples, which included male and female specimens, were treated previously by enucleating the eyes and removing the skin and most of the muscles. After this, the samples were immersed in hot hydrogen peroxide (130 vol, Mazzochini, Caxias do Sul, Brazil) at 80ºC for 2 h and maintained at 37ºC for 48 h to remove the remnants of organic tissue. Subsequently, the samples were washed with distilled water and dried.
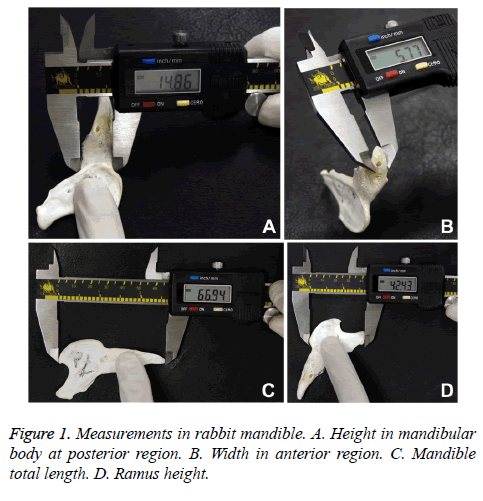
The measurements were taken using a digital calliper (Bull Tools® 0-150 mm, Echain, Taichung, Taiwan) in the following regions (Figures 1 and 2):
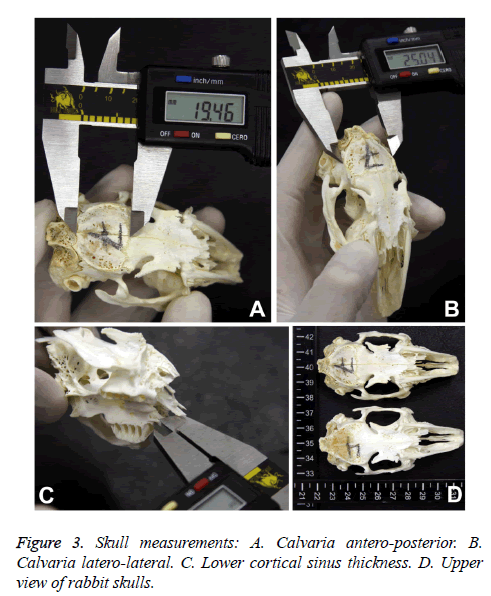
- Calvaria: Latero-lateral, antero-posterior and its thickness.
- Body of mandible divided into three regions, measuring the maximum height and thickness:
Anterior region: posterior to central incisors
Middle region: anterior to first premolar.
Posterior region: posterior to third molar.
- Ramus of mandible by side: maximal length, height and thickness.
- Total mandible length.
- Lower cortical sinus thickness.
The measurements of each region were organized in tables, recording the mean, standard deviation and confidence interval. In the morphometric analysis of mandible, the height and width of the three assessed regions (anterior, middle and posterior) were compared. For the height, the Kruskal-Wallis test was used, with a Tukey post-test (p=0.05), due to the nonnormal distribution. The ANOVA test with the Holm-Sidak post-test (p=0.05) was applied in the analysis of the width, due to the normal distribution of this data.
The morphometric parameters of calvaria (latero-lateral, antero-posterior and thickness) were recorded in boxplot graphs to help understand the data distribution.
Results
The mean, standard deviation and confidence interval of mandibular body, ramus and calvaria were summarized in Tables 1-3, respectively.
| Measures | Mean | S.D. | CI |
|---|---|---|---|
| AR height | 6.2 | 0.4 | 5.9 to 6.5 |
| AR thickness | 4.7 | 0.1 | 4.5 to 4.9 |
| MR height | 12.5 | 0.8 | 12 to 13 |
| MR thickness | 6.6 | 0.3 | 6.4 to 6.8 |
| PR height | 15.9 | 1 | 15.3 to 16.5 |
| PR thickness | 4.7 | 0.2 | 4.6 to 4.8 |
Table 1. Mean, standard deviation and 95 confidence interval (CI) of height and thickness of mandibular body in the Anterior (AR), Middle (MR) and Posterior Regions (PR).
| Measures | Right | Left | ||||
|---|---|---|---|---|---|---|
| Mean | S.D. | CI | Mean | S.D. | CI | |
| Length | 30.1 | 1.2 | 29.3 to 30.9 | 29.7 | 1.4 | 28.8 to 30.6 |
| Height | 44 | 1.3 | 43.2 to 44.8 | 43.8 | 1.1 | 43.1 to 44.5 |
| Thickness | 1.3 | 0.1 | 1.2 to 1.4 | 1.4 | 0.1 | 1.3 to 1.5 |
Table 2. Mean, standard deviation and 95 confidence interval (CI) value by side of length, height and thickness mandibular ramus.
| Measures | Mean | S.D. | CI |
|---|---|---|---|
| Lateral | 26 | 0.8 | 25.5 to 27.5 |
| Antero-posterior | 18.2 | 1 | 17.6 to 18.8 |
| Thickness | 1.5 | 0.2 | 1.4 to 1.6 |
Table 3. Mean, standard deviation and 95 confidence interval (CI) of the different measures performed in calvaria.
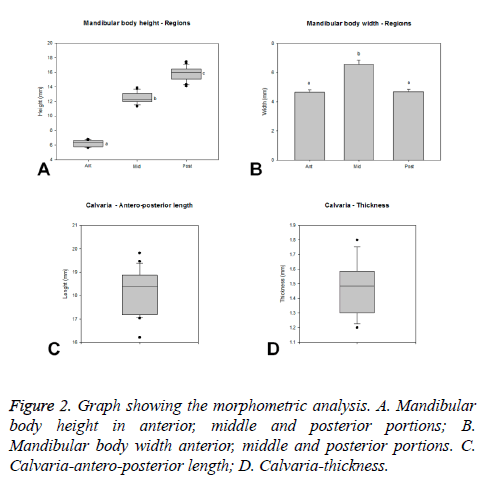
The measurements of total body of mandible showed a mean of 67.2 (±2 mm) with a Confidence Interval (CI) of 66 to 68.4 mm (Figure 1C). The mandible body had significant heights differences (p<0.05) in the three portions evaluated (medians: anterior-6.32 mm; middle-12.2 mm; posterior-16.02 mm), being possible to identify increasing values from the anterior to posterior regions (Figure 2A).
The width of the mandible body presented similar values (p>0.05) in the anterior (4.66 ± 0.18 mm) and posterior (4.69 ± 0.17 mm) regions. The middle portion (6.58 ± 0.27 mm) presented significant higher width (p<0.05) compared to the other portions (Figure 2B).
The calvaria of rabbits presented a laterolateral length ranging from 25.04 to 27.38 mm with a median of 25.95 mm (Q1-25.28 mm, Q3-26.72 mm). The anteroposterior length ranged from 16.21 to 19.82 mm with median of 18.4 mm (Q1-17.19 mm, Q3-18.87 mm, Figure 2C). Thus, the laterolateral length predominates over the anteroposterior parameter. The calvaria thickness presented a median of 1.49 mm (Q1-1.3 mm, Q3-1.59 mm, Figure 2D), ranging from 1.2 to 1.8 mm.
The lower cortical thickness for approaching to maxillary sinus exhibited a mean of 1.1 (± 0.1 mm) with a CI from 1 to 1.2 mm (Figure 3).
Discussion
Animal model studies have been widely used in the literature to investigate bone regeneration through different bone grafts, testing these bone substitutes in regions such as the calvaria and mandibles of rabbits, rats, pigs and dogs [12-15]. However, the amount of bone formation could not be compared because the defects are made in specific sites not comparable between species.
Some studies have tested biomaterials in critical defects of calvaria [6,16,17]; i.e., those that do not heal spontaneously. Some studies have reported sufficiently large calvaria for the creation of four 8 mm defects each [17-19]. However, if two defects in the anteroposterior direction, with a safety margin of 1 mm between them, theoretically measure 17 mm, there may be a bias if the intracranial suture distance is not respected, as the mesenchymal cells can influence the results and behavior of the defects [20,21], without necessarily depending on the biomaterials tested. In this study, the average anteroposterior length of rabbit calvaria was 18.2 mm and 26 mm in a laterolateral direction, which may limit the creation of four critical size defects, because in the anteroposterior at least 19 mm is required, not including the sutures, considering 1 mm between the frontal-parietal or coronal suture, 1 mm between the defects, and 1 mm from the parietal-occipital or lambdoid suture. Therefore, the size of the rabbit calvaria and the distance of the sutures in the specimens should be assessed prior to creating the defects. Another important factor is the intraoperative drilling accidents of the brain with the trephine bur during the preparation of the defect, so an average thickness of approximately 1.5 mm should be considered.
The experimental model of external transcutaneous sinus lift in rabbits has been widely described in the literature [22,23], and was first described by Watanabe et al. [24]. They reported the low cost and easy distinction of membrane perforation among others as advantages of this model. However, a few studies performed an internal approach [25], which mainly focused on studying the response of various bone graft materials, regardless of the maxillary sinus floor thickness, where surgical windows of different sizes were made [26]. In our study, the thickness of the maxillary sinus floor has an average of 1.1 mm, and it is suggested that this be taken into consideration at the time of surgery.
Related to studies on the mandible ramus, the 44 mm in height observed in this study are similar to the values reported by Abreu et al. [7] (39 mm). However, Monfared [10] observed an average of 29 mm in ramus height, identifying a large discrepancy between these values. Some authors have reported research using ramus non-critical size defects of different sizes [27,28]. Whatever the size of the defect, an average cortical thickness of 1.3 mm should be considered, so we recommend that the defects be performed with 1.2 mm in depth and with a safety margin of 0.1-0.2 mm. In this context, the mandible ramus is a complex region to perform experiments in a rabbit model, both due to its difficult access and thin bone thickness. Therefore, the two most common areas to test biomaterials in the mandible are the angle and body [29].
In relation to the dimensions of the mandibular body, an average of 67 mm was identified as being less than the 75 mm reported by Monfared [10] in a sample of eleven rabbits. The greatest bone thickness in the mandibular body was found in the middle region, anterior to the first molar, which is ideal for the placement and study of dental implants due to its size. Shah et al. [29] concurred with this statement, describing that this area allows an easy surgical approach. The anterior and posterior region showed a similar thickness of 4.7 mm. The bone height increased posteriorly, mainly due to the mandible’s anatomy. Eleftheriadis et al. [30] created defects of 8 mm long × 3 mm wide × 3 mm deep in the middle region of the mandible, proving this to be a safe area to perform surgeries. However, Shah et al. [29] detailed in their research that a full thickness defect of 10 mm in the molar/premolar region does not heal spontaneously and it acts as a critical size defect.
This study has some limitations, such as the measurement of anatomical structures in a single species of rabbit and the sample size, among others. However, we believe that it provides valuable information to be used for studies on a rabbit model when it is used with the confidence interval. Despite these limitations, it is possible to create an experimental environment for bone graft substitutes following a surgical bone protocol that is able to emulate clinical conditions [10]. Further studies are needed with a larger sample and in different species.
Conclusion
The limited dimensions of anatomical structures should be considered when working with critical size defects in rabbits to avoid complications or accidents during the specimen’s surgery.
References
- Kumar D, Ekanthamoorthy J, Kumar S. Study of development and applications of bioactive materials and methods in bone tissue engineering. Biomed Res India 2015; 26: 55-61.
- Beltran V, Engelke W, Prieto R, Valdivia-Gandur I, Navarro P, Manzanares MC, Borie E, Fuentes R. Augmentation of intramembranous bone in rabbit calvaria using an occlusive barrier in combination with demineralized bone matrix (DBM): a pilot study. Int J Surg 2014; 12: 378-383.
- de Almeida M, Lanata-Flores A, Olate S, Pozzer L, Cantín M, Vásquez B, Albergaria-Barbosa J. The removal torque of titanium implant inserted in dog tibia with a bone defect. Int J Morphol 2013; 31: 700-705.
- Alvira-Gonzalez J, Sanchez-Garces MA, Cairo JR, Del Pozo MR, Sánchez CM, Gay-Escoda C. Assessment of bone regeneration using adipose-derived stem cells in critical-size alveolar ridge defects: an experimental study in a dog model. Int J Oral Maxillofac Implants 2016; 31: 196-203.
- Issa JP, Gonzaga M, Kotake BG, de Lucia C, Ervolino E, Iyomasa M. Bone repair of critical size defects treated with autogenic, allogenic, or xenogenic bone grafts alone or in combination with rhBMP-2. Clin Oral Implants Res 2016; 27: 558-566.
- Borie E, Fuentes R, Del Sol M, Oporto G, Engelke W. The influence of FDBA and autogenous bone particles on regeneration of calvaria defects in the rabbit: a pilot study. Ann Anat 2011; 193: 412-417.
- de Abreu AT, Veeck EB, da Costa NP. Morphometric methods to evaluate craniofacial growth: study in rabbits. Dentomaxillofac Radiol 2006; 35: 83-87.
- Kahnberg KE. Restoration of mandibular jaw defects in the rabbit by subperiosteally implanted Teflon mantle leaf. Int J Oral Surg 1979; 8: 449-456.
- Oporto G, Fuentes R, Borie E, Del Sol M, Orsi IA, Engelke W. Radiographical and clinical evaluation of critical size defects in rabbit calvaria filled with allograft and autograft: a pilot study. Int J Clin Exp Med 2014; 7: 1669-1675.
- Monfared AL. Applied anatomy of the rabbits skull and its clinical application during regional anesthesia. Glob Veter 2013; 10: 653-657.
- Valdivia IG, Tallon VW, Carvalho PL, Lozano V, Manzanares MC. Mandible measurements and dental midline deviation after alveolar nerve transection in growing rabbits. Int J Morphol 2011; 29: 52-56.
- Aaboe M, Pinholt EM, Schou S, Hjørting-Hansen E. Incomplete bone regeneration of rabbit calvarial defects using different membranes. Clin Oral Implant Res 1998; 9: 313-320.
- Cardaropoli G, Araujo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol 2003; 30: 809-818.
- Jensen OT, Cullum DR, Baer D. Marginal bone stability using 3 different flap approaches for alveolar split expansion for dental implants: a 1-year clinical study. J Oral Maxillofac Surg 2009; 67: 1921-1930.
- Du B, Gao Y, Deng Y, Zhao Y, Lai C, Guo Z, Rong M, Zhou L. Local delivery of rhVEGF165 through biocoated nHA/coral block grafts in critical-sized dog mandible defects: a histological study at the early stages of bone healing. Int J Clin Exp Med 2015; 8: 4940-4953.
- Kim BS, Choi MK, Yoon JH, Lee J. Evaluation of bone regeneration with biphasic calcium phosphate substitute implanted with bone morphogenetic protein 2 and mesenchymal stem cells in a rabbit calvarial defect model. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 120: 2-9.
- Kitayama S, Wong LO, Ma L, Hao J, Kasugai S, Lang NP, Mattheos N. Regeneration of rabbit calvarial defects using biphasic calcium phosphate and a strontium hydroxyapatite-containing collagen membrane. Clin Oral Implant Res 2015.
- Hwang JW, Park JS, Lee JS, Jung UW, Kim CS, Cho KS, Lee YK, Choi SH. Comparative evaluation of three calcium phosphate synthetic block bone graft materials for bone regeneration in rabbit calvaria. J Biomed Mater Res B Appl Biomater 2012; 100: 2044-2052.
- Yip I, Ma L, Mattheos N, Dard M, Lang NP. Defect healing with various bone substitutes. Clin Oral Implants Res 2015; 26: 606-614.
- Burgos-Florez FJ, Gavilan-Alfonso ME, Garzon-Alvarado DA. Flat bones and sutures formation in the human cranial vault during prenatal development and infancy: A computational model. J Theor Biol 2016; 393: 127-44.
- Whitton A, Hyzy SL, Britt C, Williams JK, Boyan BD, Olivares-Navarrete R. Differential spatial regulation of BMP molecules is associated with single-suture craniosynostosis. J Neurosurg Pediatr 2016.
- Perez AC, Cunha Junior Ada S, Fialho SL, Silva LM, Dorgam JV. Assessing the maxillary sinus mucosa of rabbits in the presence of biodegradable implants. Braz J Otorhinolaryngol 2012; 78: 40-46.
- Swibel Rosenthal LH, Benninger MS, Stone CH, Zacharek MA. Wound healing in the rabbit paranasal sinuses after Coblation: Evaluation for use in endoscopic sinus surgery. Am J Rhinol Allergy 2009; 23: 360-363.
- Watanabe K, Niimi A, Ueda M. Autogenous bone grafts in the rabbit maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999; 88: 26-32.
- Rahmani M, Shimada E, Rokni S, Deporter DA, Adegbembo AO, Valiquette N, Pilliar RM. Osteotome sinus elevation and simultaneous placement of porous-surfaced dental implants: a morphometric study in rabbits. Clin Oral Implants Res 2005; 16: 692-699.
- Kim YS, Kim SH, Kim KH, Jhin MJ, Kim WK, Lee YK, Seol YJ, Lee YM. Rabbit maxillary sinus augmentation model with simultaneous implant placement: differential responses to the graft materials. J Periodontal Implant Sci 2012; 42: 204-211.
- Yang C, Liu Y, Li C, Zhang B. Repair of mandibular defects by bone marrow stromal cells expressing the basic fibroblast growth factor transgene combined with multi-pore mineralized Bio-Oss. Mol Med Rep 2013; 7: 99-104.
- Yang J, Kang Y, Browne C, Jiang T, Yang Y. Graded porous ß-tricalcium phosphate scaffolds enhance bone regeneration in mandible augmentation. J Craniofac Surg 2015; 26: 148-153.
- Shah SR, Young S, Goldman JL, Jansen JA, Wong ME. A composite critical-size rabbit mandibular defect for evaluation of craniofacial tissue regeneration. Nat Protoc 2016; 11: 1989-2009.
- Eleftheriadis E, Leventis MD, Tosios KI, Faratzis G, Titsinidis S, Eleftheriadi I, Dontas I. Osteogenic activity of ß-tricalcium phosphate in a hydroxyl sulphate matrix and demineralized bone matrix: a histological study in rabbit mandible. J Oral Sci 2010; 52: 377-384.