Research Article - Biomedical Research (2017) Volume 28, Issue 10
Microstructural analysis using X-ray diffraction after fluoride administration
Seoul Hee Nam and Hye-Young Kim*
Department of Dental Hygiene, College of Health Science, Kangwon National University, Samcheok-si, 25949, Republic of Korea
- *Corresponding Author:
- Hye-Young Kim
Department of Dental Hygiene
College of Health Science
Kangwon National University, Republic of Korea
Accepted date: March 08, 2017
Abstract
The amount of fluoride found in the bone is usually correlated with the amount of fluoride found either in water or in a person’s diet. The purpose of this study was to evaluate the beneficial incorporation of fluoride into rat bone after prenatal fluoride administration, using microstructural analysis. Fifteen 8- week-old female were divided into five groups (one control group and four experimental groups), with three rats in each group. During the mating period, pure redistilled water (containing no fluoride) was given to the control group, and redistilled water containing 1/10/20/100 ppm sodium fluoride was given to the experimental groups. The drinking water was prepared by dissolving 2.2 mg NaF (Yakuri Pure Chemicals Ltd., Osaka, Japan) per liter of redistilled water. Rats were sacrificed 4 weeks after their delivery, and their femurs were extracted. X-ray diffraction (XRD; electromagnetic waves) and Fourier transform infrared spectroscopy (FTIR) were performed. The change in the hydroxyapatite structure to fluoroapatite was not confirmed by XRD, and FTIR showed that the inorganic element bands (carbonate, phosphate, and amide band) seemed to be slightly higher in the 20 ppm group, suggesting that the prenatal fluoride treatment through the incorporation of fluoride ions into the hydroxyapatite crystals affected the unit cell size and the bone microstructure.
Keywords
Electromagnetic radiation, Fluorides, Fourier transform infrared spectroscopy (FTIR), Hydroxyapatites, Xray diffraction (XRD; electromagnetic waves)
Introduction
Despite its link with dental fluorosis, fluoride has been used all over the world for the past 70 years to prevent dental caries. Fluoride is important for body tissue mineralization [1]; when an appropriate amount of it is consumed, bone and tooth integrity can be boosted, thus promoting oral and general health. As such, fluoride is regarded by many as the most effective dental caries preventive agent [2]. In addition, water/ milk/salt fluoridation is considered advantageous, and the use of fluoride tablets and the topical application of fluoride preparations on the tooth surfaces are recommended [3-5].
Fluoride has a high affinity with calcified tissues, and is concentrated in the dental and bone structures [6]. Approximately 99% of the fluoride in the body is associated with calcified tissues, where it mainly substitutes for the hydroxyl group (OH-) of the hydroxyapatite (HA) crystals (Ca10[PO4]6[OH]2) [7,8]. The sizes, shapes, and arrangement of the apatite crystals in the bone are seen as the major determinants of the biomechanical properties of fluoride [8]. Fluoride can also affect the putative precursor phases in bone mineral apatite formation [9]. It has been reported that the primary teeth have better crystal formation and a generally better morphology when fluoride supplementation begins early in pregnancy [10,11]. There is no information currently available, however, showing a relation between the tooth fluoride concentration and the tooth (dentin and enamel) crystallite size.
Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) have been used for characterizing apatite [12,13]. It was shown in some reports that the XRD patterns of bovine [12] and rodent [13,14] bone apatites were sharpened or better resolved with an increase in fluoride content, and the same reports concluded that the in-vivo improvement of the bone apatite crystallinity through fluoride ingestion leads to a more stable crystal system. It has been shown in previously reported FTIR and XRD studies that the increase in the percentage of fluoride in bone ash is accompanied by a sharpening of the corresponding diffraction pattern [12,15,16]. The increase in crystallinity with fluoride is due to the formation of larger and/or more perfect crystals, but comparatively less is known about the influence of certain factors on apatite in vivo.
It is mainly due to the strong affinity of fluoride with the apatitic mineral phase in the skeletal tissue that nearly all the fluoride ingested and retained by the body is deposited in such tissue [17]. Fluoride is mostly concentrated in the bone tissue areas undergoing active mineralization, where it easily incorporates into the hydroxide (OH) lattice sites during the nucleation and growth of the apatite crystals. The amount of phosphate (PO4, one of the two major lattice components) in the bone is not at all affected by fluoride, and the calcium concentration increases minimally, if at all.
Grynpas and Rey [9] demonstrated that no difference in bone crystal size/strain with fluoride treatment was revealed by XRD. It was found, however, that the phosphate bands shifted, which is probably due to the unit cell size change induced by the fluoride ion and measured via FTIR. The recent adult bone mineral XRD profiles suggest that the uptake of fluoride is accompanied by substantial increases in the apatite crystal size and/or lattice perfection [12,15,18]. Most interestingly, it was observed that these changes associated with fluoride did not affect all the external crystal dimensions but were restricted to crystal width and/or thickness increases, with no measurable change in the crystal length. This size anisotropy is most evident in the bones of adult individuals who have taken in large amounts of fluoride from drinking water for long periods of time [15]. Despite the fact that a similar effect has been reported for the cortical bone apatite of osteoporotic patients after long-term fluoride treatment [18], it is currently not clear if this anisotropic fluoride effect has a physicochemical or metabolic origin. The sizes, shapes, and arrangements of the apatite crystals in the bone are the major determinants of the biomechanical properties of fluoride, and any alteration in these texture properties of bone apatite can contribute to the deleterious clinical features of skeletal disorders like osteogenesis imperfection [19]. Zipkin et al. [20] showed an inverse relationship between the citrate and fluoride contents of human bones, and Posner et al. [15] suggested, based on the XRD analysis results that they obtained, that the improved crystallinity and the isomorphous substitution of fluoride in the apatite structure produce a more stable bone apatite. Eanes and Hailer [17], after observing adult human bone apatites, suggested that fluoride stimulates seed crystal growth in a similar anisotropic manner, which in turn suggests that the latter growth in vivo was partly the consequence of the direct fluoride mineral interactions.
When fluoride can be actually incorporated into bone mineral is not clear, and the role of prenatal fluoride supplements has been controversial. In this study, fluoride was administered to rats prenatally to measure the fluoride concentration in the bone and to determine the effect of fluoride on the bone structure as well as the effects of the placental transfer of fluoride.
Materials and Methods
Animal control
Fifteen 8-week-old female rats initially weighing 180-200 g on average were divided into five groups (one control group and four experimental groups), with three rats in each group. They were housed in separate cages in a room that was used exclusively for this study, and were mated 1:1 with 10-week-old male rats initially weighing 300-320 g on average, which were introduced to the female rats’ cages and were made to cohabit such cages with the female rats for 3 weeks. During the mating period, pure redistilled water (containing no fluoride) was given to the control group, and redistilled water containing 1/10/20/100 ppm sodium fluoride was given to the experimental groups. The drinking water was prepared by dissolving 2.2 mg NaF (Yakuri Pure Chemicals Ltd., Osaka, Japan) per liter of redistilled water. The climate-controlled room, which had 12-hour light/dark cycles, had a 20 ± 2°C temperature and 40-80% humidity. The 15 pregnant rats were separated a few days before delivery, after which the litters were kept with their dams for 3 weeks, were then separated from their dams, and were thereafter housed without their dams for 1 week.
Preparation of specimens
The young rats were sacrificed 4 weeks after their delivery. Their right and left femurs were extracted, and all the adhering muscle tissues were completely scraped off. The femurs were then ashed at 500°C for 2 hours in porcelain crucibles in a thermo-control furnace (Type 212, Mortia©, Japan), for microstructural analysis.
Microstructural analysis
XRD: The powders of the left femurs were dried for analysis using an X-ray diffractometer (X'Pert APD, Philips, Netherlands). A cobalt target was used with 40 kV and 40 mA voltage and current settings, respectively. All the samples were examined from 10 to 70° 2θ at a 1°/min 2θ scanning speed and a 0.03° 2θ step size. The energy of the X-ray photon was related to the radiation frequency by E=hv, where:
h=Planck’s constant (6.626 × 10-34J/s or 4.135 × 10-15eV/s) and
v=frequency.
The wavelength λ was related to the photon energy by λ=1.240 × 10-6/E.
FTIR: The left femurs were examined via FTIR (Bruker IFS 66/FRA 106, Bruker, Germany). A mixture of 1 mg of the powdered sample and 2 mg of KBr was pelletized under vacuum. The pellets were analyzed in the 400-4,000 cm-1 range by 128 scans.
Results
Microstructural analysis
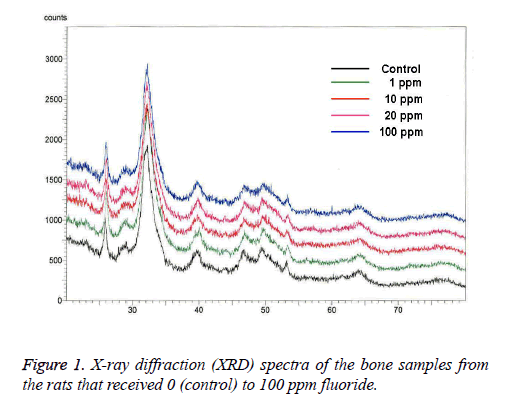
XRD: The broad-range XRD spectra were in accordance with the typical hydroxyapatite spectra. The XRD analysis revealed that there were no changes in rat bone apatite crystallinity after prenatal fluoride administration (Figure 1).
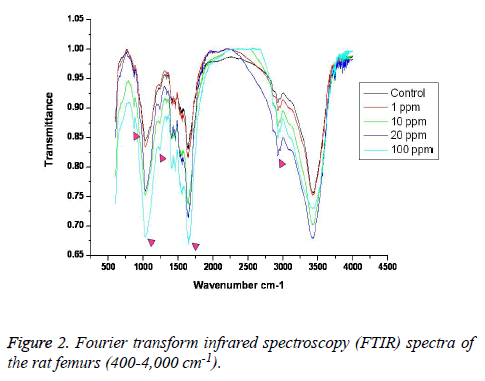
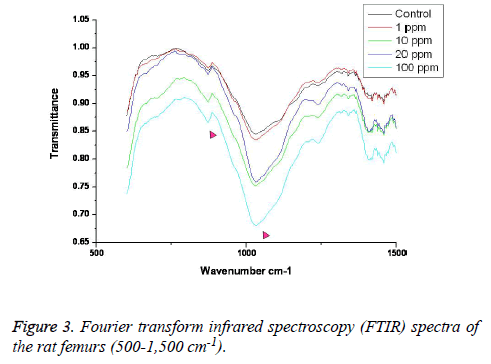
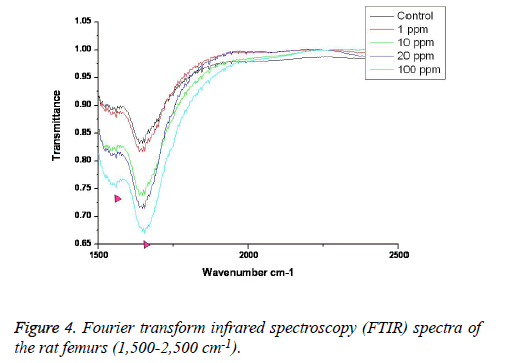
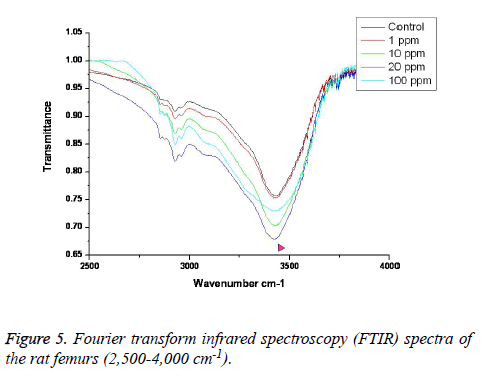
FTIR: The overall FTIR spectral features of the femoral bones showed the typical vibration modes of apatite (Figure 2), although there were spectral variations in the relative height and breadth of the carbonate, phosphate, and amide bands compared to the sites in the control. The spectra were characterized by the transmittance attributed to the CO32- and PO43- vibrations of the extracellular matrix’s mineral and amide (I, II, and III) vibrations. Bands were found at 878, 871, and 866 cm-1, respectively, and the high, sharp peaks were dependent on the fluoride concentration. The phosphate vibrational regions generally exhibited a broad band in the 900-1,200 cm-1 region (Figure 3). Phosphate bands were also found at these peak areas, and the high, sharp peaks were dependent on the fluoride concentration. The amide I, II, and III contours were attributed primarily to C=O (amide I) and to the collagen matrix’s combined N-H and C-N (amide II and III) vibrations. Amide I and II and/or amide III bands were found in the 1,400-1,700 cm-1 region and at 3,420 cm-1, and the high, sharp peaks were dependent on the fluoride concentration (Figures 4 and 5).
Discussion
The use of fluoride and fluoridation for dental caries prevention is recognized by over 90 professional health organizations as the most effective dental public health measure at present [21,22], but there is little indication that the use of fluoride prenatally will benefit the developing fetus systemically. In this study, microstructural analysis was used to determine if the prenatal administration of fluoride (through the placenta) increases the beneficial concentration of fluoride in rat bone, and to analyze its effect, if any, on the bone structure. The increase in crystallinity observed in the bone formed during the time of fluoride consumption was on account of the formation of larger and/or more perfect crystals. The substitution of fluoride for hydroxyl ions produces a structure that is less soluble in water and less labile to chemical attack. It may be pointed out, by analogy, that fluoridated enamel and dentine may be less labile to the specific biochemical attacks resulting in dental caries due to the stabilizing influence of improved crystallinity and fluoride substitution [15]. In the study conducted by Grynpas and Rey [9], when XRD was used, no difference was shown in the bone crystal size and strain after fluoride treatment, but when FTIR was used, increased crystallinity was found in the fluoride-fed animals, which seemed to have been associated with the decrease of the labile phosphate environment. In this study, the XRD analysis showed no changes in the rat bone apatite crystallinity after prenatal fluoride administration.
FTIR can provide information about the size/perfection of the bone mineral apatite crystals as well as the relative carbonate, phosphate, and mineral:matrix ratios of specimens at discrete anatomical locations. In this study, FTIR offered structural information on the local environments of the phosphate and carbonate ions, etc. Changes in the phosphate environments were observable in the PO4 bands in the 900-1,200 cm-1 region. Important changes were also apparent in the CO3 bands and were revealed by a shoulder at 878, 871, and 866 cm-1. The high, sharp peaks were dependent on the fluoride concentration. The broad carbonate vibrational region is typically composed of three overlapping bands attributable to the CO3 substituted for the OH, PO4, and labile carbonate positions in the apatite lattice [23]. Additionally, amide I, II, and III bands were found in the 1,400-1,700 cm-1 region and at 3,420 cm-1, and the high, sharp peaks were dependent on the fluoride concentration.
As mentioned earlier, higher peak intensities appeared in the prenatal-fluoride-administration groups than in the control group, but in the 100 ppm group, there were no significant changes compared with the control group. These ion-related peaks have been shown to possess a strong affinity with these sites, and to displace all other anions [9]. The increase of this band in fluoridated bone may be attributed to the fluoride ion interactions in each unit cell. Such band displacement is frequently observed in minerals, and is generally attributed to the unit cell volume variations. This interpretation seems consistent with the well-known effect of fluoride ions on the apatitic-lattice dimensions [24]. In the bone with low fluoride levels, this band has been assigned to an unstable environment of carbonate ions in a perturbed area of the crystal, probably near its surface [25]. In prenatal fluoridated apatites, however, an intense band has been noticed in this position, which has been attributed to the interaction between the fluoride and carbonate ions. The data presented in this study were similar to the results obtained by Grynpas and Rey [9] and Vignoles et al. [26].
Many studies have been conducted on the placental transfer of fluoride, and it has been the subject of controversy [27-30]. Based on the study results obtained so far, it is assumed that the placenta has a partial blocking effect, allowing the passage of only limited amounts of the fluoride given to a pregnant mother. These results, however, demonstrated the higher crystallinity of bone mineral after prenatal fluoride treatment, with the exception of the high fluoride concentrations in the 100 ppm group. It was noted that this amount of prenatal fluoride did not change the bone morphology. The results of this study confirmed that the incorporation of fluoride in carbonate, phosphate, and amide cells could increase the abilities of these unit cell structures to bond with other elements.
Conclusion
The X-ray diffraction (XRD) analysis that was performed in this study showed no changes in the rat bone apatite crystallinity after prenatal fluoride administration. In Fourier transform infrared spectroscopy (FTIR), the carbonate, phosphate, and amide I, II, and/or III bands showed high, sharp peaks. This effect was dependent on the fluoride concentration and seemed to have been associated with the interaction of the fluoride ions in the apatite lattice, suggesting that the prenatal fluoride treatment through the incorporation of fluoride ions into the hydroxyapatite crystals affected the unit cell size and the bone microstructure.
References
- Browne D, Whelton H, O'Mullane D. Fluoride metabolism and fluorosis. J Dent 2005; 33: 177-186.
- Zimmer S, Jahn KR, Barthel CR. Recommendations for the use of fluoride in caries prevention. Oral Health Prev Dent 2003; 1: 45-51.
- Hawkins R, Noble J, Locker D. A comparison of the costs and patient acceptability of professionally applied topical fluoride foam and varnish. J Public Health Dent 2004; 64: 106-110.
- Seppä L. Fluoride varnishes in caries prevention. Med Princ Pract 2004; 13: 307-311.
- Verma RJ, Sherlin DM. Hypocalcaemia in parental and F1 generation rats treated with sodium fluoride. Food Chem Toxicol 2002; 40: 551-554.
- National Research Council. Health effects of ingested fluoride. National Academy Pres, Washington DC, 1993.
- LeGeros RZ, Craig RG. Strategies to affect bone remodeling: osteointegration. J Bone Miner Res 1993; 8 Suppl 2: S583-596.
- Vieira A, Hancock R, Limeback H, Schwartz M, Grynpas M. How does fluoride concentration in the tooth affect apatite crystal size? J Dent Res 2003; 82: 909-913.
- Grynpas MD, Rey C. The effect of fluoride treatment on bone mineral crystals in the rat. Bone 1992; 13: 423-429.
- Glenn FB, Glenn WD, Duncan RC. Prenatal fluoride tablet supplementation and improved molar occlusal morphology : part V. J Dent Child 1984; 51: 19-23.
- Groeneveld A, Van Eck AA, Backer Dirks O. Fluoride in caries prevention: is the effect pre- or post-eruptive? J Dent Res 1990; 69 Spec No: 751-755.
- Zipkin I, Eanes ED, Shupe JL. Effect of Prolonged Exposure to Fluoride on the Ash, Fluoride, Citrate, and Crystallinity of Bovine Bone. Am J Vet Res 1964; 25: 1591-1597.
- Menczel J, Posner AS, Schraer H. Comparative fixation of Sr89 and Ca45 by calcified tissues as related to fluoride induced changes in crystallinity. Proc Soc Exp Biol Med 1962; 110: 609-613.
- Schraer H, Posner AS, Schraer R, Zipkin I. Effect of fluoride on bone "crystallinity" in the growing rat. Biochim Biophys Acta 1962; 64: 565-567.
- Posner AS, Eanes ED, Harper RA. X-ray diffraction analysis of the effect of fluoride on human bone apatite. Arch Oral Biol 1963; 168: 549-570.
- Eanes ED, Zipkin I, Harper RA, Posner AS. Small-angle x-ray diffraction analysis of the effect of fluoride on human bone apatite. Arch Oral Biol 1965; 10: 161-173.
- Eanes ED, Hailer AW. The effect of fluoride on the size and morphology of apatite crystals grown from physiologic solutions. Calcif Tissue Int 1998; 63: 250-257.
- Baud CA, Very JM, Courvoisier B. Biophysical study of bone mineral in biopsies of osteoporotic patients before and after long-term treatment with fluoride. Bone 1988; 9: 361-365.
- Vetter U, Eanes ED, Kopp JB, Termine JD, Robey PG. Changes in apatite crystal size in bones of patients with osteogenesis imperfecta. Calcif Tissue Int 1991; 49: 248-250.
- Zipkin I, McClure FJ, Lee WA. Relation of the fluoride content of human bone to its chemical composition. Arch Oral Biol 1960; 2: 190-195.
- Hutton WL, Linscott BW, Williams DB. The Brantford fluorine experiment. Interim report after five years of water fluoridation. Can J Public Health 1951; 42: 81-87.
- Bratthall D, Hänsel-Petersson G, Sundberg H. Reasons for the caries decline: what do the experts believe? Eur J Oral Sci 1996; 104: 416-422.
- Rey C, Collins B, Goehl T. The carbonate environment in bone mineral: a resolution-enhanced fourier transform infrared spectroscopy study. Calcif Tissue Int 1989; 45: 157-164.
- Larsen MJ, Jensen SJ. Solubility, unit cell dimensions and crystallinity of fluoridated human dental enamel. Arch Oral Biol 1989; 34: 969-973.
- Rey C, Shimizu M, Collins B. Resolution-enhanced Fourier transform infrared spectroscopy study of the environment of phosphate ions in the early deposits of a solid phase of calcium-phosphate in bone and enamel, and their evolution with age. I: Investigations in the upsilon 4 PO4 domain. Calcif Tissue Int 1990; 46: 384-394.
- Vignoles M, Bonel G, Holcomb DW. Influence of preparation conditions on the composition of type B carbonated hydroxyapatite and on the localization of the carbonate ions. Calcif Tissue Int 1988; 43: 33-40.
- Clarkson JJ, McLoughlin J. Role of fluoride in oral health promotion. Int Dent J 2000; 50: 119-128.
- Driscoll WS. A review of clinical research on the use of prenatal fluoride administration for prevention of dental caries. ASDC J Dent Child 1981; 48: 109-117.
- Glenn FB, Glenn WD 3rd, Burdi AR. Prenatal fluoride for growth and development: Part X. ASDC J Dent Child 1997; 64: 317-321.
- Toyama Y, Nakagaki H, Kato S. Fluoride concentrations at and near the neonatal line in human deciduous tooth enamel obtained from a naturally fluoridated and a non-fluoridated area. Arch Oral Biol 2001; 46: 147-153.