Review Article - Journal of Cholesterol and Heart Disease (2017) Volume 1, Issue 1
MicroRNAs and Myocardial Infarct: Investigating the Controversial Role of Second Generation Biomarkers.
Athar Khalil#, Amina Kamar#, Georges Nemer*
Department of Biochemistry and Molecular Genetics, American University of Beirut, Beirut, Lebanon
#The authors contributed equally to the work
- Corresponding Author:
- Georges Nemer
Department of Biochemistry and Molecular Genetics American University of Beirut, Beirut, Lebanon
Tel: 9611350000 (4876)
E-mail: gn08@aub.edu.lb
Accepted date: April 18, 2017
Citation: Khalil A, Kamar A, Nemer G. MicroRNAs and Myocardial Infarct: Investigating the Controversial Role of Second Generation Biomarkers. J Cholest Heart Dis. 2017;1(1):8-13
Abstract
Myocardial infarction (MI) is a major manifestation of coronary heart disease that causes cardiac tissue necrosis, myocardial inflammation, pathological remodeling and dysfunction of the left ventricle. It is thus very critical to diagnose myocardial infarction in chest pain patients as soon as possible. The golden standard for diagnosing acute myocardial infarction is still cardiac troponin I (cTnI) which is not a very sensitive or specific assay. Therefore, detecting specific and accurate biomarkers at early stage is a necessity for optimal diagnosis, prognosis, prevention and treatment. miRNAs have proved to be good biomarkers in MI, in this review we will focus on four miRNAs (miRNA-133,-208,-1,and -499) that are intensively studied as biomarkers in AMI patients. Most studies have revealed that these micro-RNAs show low or no expression levels in the plasma of healthy individuals, while they are released into peripheral circulation in time dependent manner in AMI patients. This gave them the power to be used as a diagnostic tool for AMI. Additionally, some miRNAs were used for the prediction of future cardiovascular death as follow up studies have publicized.
Cardiovascular disease (CVD) is the major cause of mortality and morbidity in developed countries [1]. By 1990, the total number of deaths caused by CVD (mostly coronary heart disease, stroke and rheumatic heart disease) was 14.4 million. By 2005, this number had globally ascended to 17.5 million. The World Health organization (WHO) estimated that by the end of 2015, CVDs had accounted for 30 percent of all deaths worldwide which constituted about 20 million deaths [2].
The coronary artery network is a system of blood vessels that nourish the cardiac muscle. A proper coronary blood circulation is needed to maintain heart homeostasis after birth. During human embryonic coronary blood vessel formation, the cardiac epicardium plays an essential role by supplying cells to the coronary vasculature. Abnormal epicardium formation leads to various congenital anomalies of the heart wall and the coronary vascular system. Thus, any change in the coronary function leads to myocardial ischemia, infarction and cardiac failure [3]. Cardiac pathologies are associated with aberrations in the expression profile of genes that are essential for the heart function [1]. Stable angina pectoris, unstable angina pectoris, myocardial infarction (MI), heart failure (HF) and sudden death are five major manifestations of coronary heart disease. Acute coronary syndromes (ACS) comprise unstable angina, non-STelevation MI (nSTEMI), ST-elevation MI (STEMI) and sudden cardiac death.
Myocardial infarction leads to deficient blood supply in addition to oxidative stress. This results in cardiac tissue necrosis, myocardial inflammation, pathological remodeling and a dysfunction of the left ventricle. Additional injury is caused by early reperfusion of the ischaemic section by thrombolytic treatment or surgical revascularization, although these processes are still considered effective for recovering cardiac damage [4].
MI Diagnosis
According to WHO European Myocardial Infarction, MI diagnosis is based on clinical history, electrocardiogram (ECG), and enzyme quantifications in blood and postmortem outcomes [5]. Creatine kinase– MB isoenzymes, cardiac myoglobin, and troponins are current AMI biomarkers that have been commonly used in clinical diagnosis [6]. The gold standard for diagnosing acute myocardial infarction is still cardiac troponin I (cTnI), but its plasma concentrations are not very sensitive and accurate. A false positive result might appear in non-cardiac diseases such as chronic kidney disease, severe sepsis and septic shocks [7]. Thus detecting specific and accurate biomarkers is a necessity for an optimal diagnosis, prognosis, prevention and treatment in MI.
Many recent studies have reported the existence and the prominence of circulating miRNAs in the plasma. Those miRNA are stable since they are protected in microparticles (including exosomes, microvesicles, and apoptotic bodies) and can form miRNA-protein complexes that are resistant to degradation [8,9]. In addition, circulating miRNAs are highly specific and selective with prolonged half-life, and are able to differentiate between pathologies. Thus, circulating miRNAs fulfill a number of criteria that allow them to be considered as ideal clinical biomarkers that can be quantified by real-time- PCR or microarrays [9,10].
miRNA formation and origin in circulation
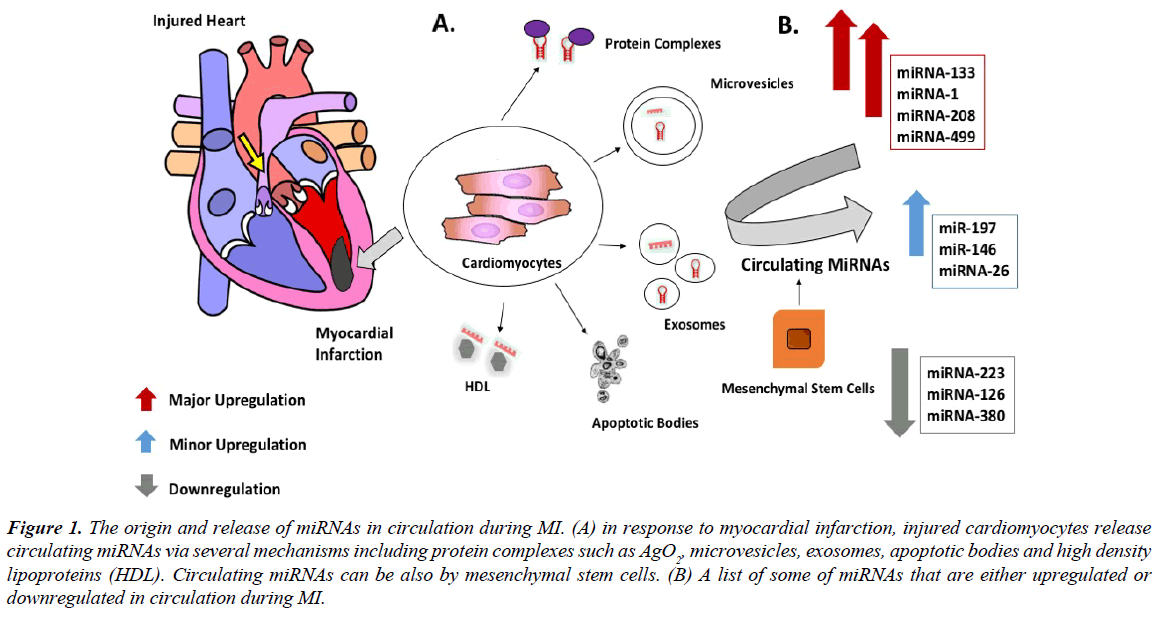
A microRNA (miRNA) is an evolutionary conserved small non-coding RNA molecule of ~22 nucleotides in length presented in animal genome [11]. miRNAs are involved in many molecular processes mainly regulation of gene expression across species. Initially, miRNA are transcribed as pri-mRNA (long primary RNA). The latter is processed into ~70 bp stemloop precursor miRNA (pre-miRNA) by RNase III enzyme Drosha (also known as ribonuclease 3). Pre-miRNAs are then transported to the cytoplasm by exportin-5 and are subsequently cleaved by cytoplasmic endoribonuclease Dicer to release mature miRNA11. The binding of miRNA/Argonaute protein complexes to an mRNA induces either direct inhibition of protein translation or mRNA degradation. It is not very clear how miRNAs are released from the cell. It is proposed that cytoplasmic miRNAs could be released by microvesicles, which is exported through blebbing of cytoplasmic membrane. Those miRNAs are present in circulation as free microparticles which could be linked to high-density lipoprotein (HDL) or attached to RNA-binding proteins such as Ago 28 [12]. It was also shown that mesenchymal stem cells can release miRNA containing exosomes which are released as pre-miRNAs and not in their mature form [13]. Another study as showed that miRNA secretion is dependent on ceramide [14]. Additional studies are required to determine the real mechanisms that control why some miRNAs are released into exosomes and others are trapped within the cell (Figure 1).
Figure 1: The origin and release of miRNAs in circulation during MI. (A) in response to myocardial infarction, injured cardiomyocytes release circulating miRNAs via several mechanisms including protein complexes such as AgO2, microvesicles, exosomes, apoptotic bodies and high density lipoproteins (HDL). Circulating miRNAs can be also by mesenchymal stem cells. (B) A list of some of miRNAs that are either upregulated or downregulated in circulation during MI.
During embryogenesis, miRNAs play pivotal roles in cardiac development including cardiomyocyte differentiation and proliferation. However, circulating miRNA in whole blood and plasma are highly associated with diverse forms of cardiovascular disease such as coronary artery disease, myocardial infarction, heart failure and cardiac death [15]. MiRNA can be released by fibroblasts, endothelial and cardiac cells. For example, miR-499 and miR-208a are specifically expressed in the heart [16], while muscle-rich miRs such as miR-1 and miR-133a/b were shown to be expressed in addition to cardiomyocytes in the skeletal muscle cells [17]. However, they are still proposed as potential biomarkers for AMI. Thus, in the context of myocardial injury, the release of unique miRNA proposes process specificity [18].
miRNA as novel cardiovascular disease biomarkers
In cardiovascular disease, circulating microRNAs (miRNAs) are considered as possible disease-specific biomarkers. Numerous studies and animal models have examined their diagnostic value with respect to coronary artery disease (CAD) and myocardial infarction (MI). Moreover, circulating miRNAs prognostic capabilities in risk stratification of future disease have been assessed. Numerous miRNAs are defined to complement protein-based biomarkers or classical risk factors in the diagnosis of CAD or MI. Besides, miRNAs are considered as potential new biomarkers in the discrimination of unstable angina pectoris (UAP) [19].
Recent studies have shown that the human genome includes thousands of miRNAs, 300 of which are roughly expressed in the heart [20].Cardiomyocyte-enriched miRNAs, including miR-1, miR208a, miR-208b, miR-133a, miR-133b, and miR-499 have been suggested as potential diagnostic markers in patients with acute myocardial infarction based on their tissue selectivity [6,21,22]. Moreover, some of these microRNAs could be used in the treatment process through effective approaches such as chemically modified antisense oligonucleotides, antagomir and locked nucleic acids. Thus, it is essential to conduct clinical trials on therapeutic and diagnostic potentials of miRNAs through extensive researches [23].
On the other hand, it is very critical to diagnose myocardial infarction in chest pain patients as soon as possible. Since the best diagnostic biomarkers that are used now [mainly cardiac troponins (cTns) and creatine kinase MB (CKMB)]; limitations as they might be elevated in other diseases [24,25] or they require loss of myocardial cell integrity and leakage of cardiac constituents and thus a late event in the prognosis of the infarction [26]. Thus, studies have focused on have some using miRNAs as potential diagnostic and prognostic biomarkers instead for AMI. In this review we will focus on the current knowledge of circulating miRNas as putative biomarkers in cardiovascular diseases notably on miR-133,-1,-208, and -499 as novel biomarkers of acute myocardial infarction.
miRNA-133 as a biomarker of AMI
Among the most studied miRNAs as biomarker for detecting and following up patients with AMI is miRNA133. miRNA- 133a is a muscle specific miRNA that is expressed abundantly in myocardial cells and plays important roles in myogenesis, cardiac development and hypertrophy [6]. This mi-RNA shows low expression levels in the plasma of healthy individuals, while it is released into peripheral circulation from the injured myocardium after Ca2+ stimulation. This microRNA is highly, but not specifically expressed in the heart [27].
One study done by Dao Wen Wang et al., showed an elevation of the circulating miR-133a during early phase of AMI, by which its level increases in time-dependent manner and exhibited a similar trend as cTnI. This study included 13 patients with AMI and 27 healthy volunteers, 5 subsequent blood samples were obtained at 4, 12, 24, 48, 72 hours after the first administration to the hospital, and real time-PCR was used for miRNA quantification. The pattern was similar in all patients with rapid increase at the beginning, achieving a peak at 21.6 ± 4.5 hours after the onset of AMI symptoms, and then gradually returned close to normal level on the following days [28].
Another study aimed to assess the relation between miR-133a and myocardial damage and to evaluate its prognostic value in re-perfused STEMI. miR-133a concentrations were evaluated in 216 consecutive patients with STEMI experiencing primary angioplasty less than 12 hours after symptom onset. Patients were divided into 2 groups categories defined by the average miR- 133a value on admission (miR-133<median group 97patients vs. miR-133a ≥ median group 95 patients). The primary clinical end point was the occurrence of major adverse cardiovascular events defined as a composite of death, re-infarction, and new congestive heart failure within 6 months after infarction. At 6-month follow-up, there were 15 deaths (14%) in the ≥ median miR-133a value on admission group and 5 (5%) in the miR133a b median group (P =0.014). Approximately all the death cases were due to cardiac problems, including cardiogenic shock, ventricular septal defect, recurrent infarction, sudden cardiac death and strokes. This study showed that elevated miR-133a concentrations are associated with less myocardial salvage, larger infarcts, more pronounced reperfusion injury, and left ventricular dysfunction as directly visualized by CMR [29]. However, in accordance with Widera et al., they found that miR-133a concentrations lost its independent correlation with clinical outcome upon multivariate adjustment for traditional clinical and CMR prognostic markers [30].
Other study which included 444 patients with NSTEMI or STEMI, detected a higher level of miR133a in thirty-four deaths (7.7%) during 6 months of follow-up compared to those who survived. The level of this microRNA was significantly related to all-cause mortality which remained associated with the risk of death after adjustment for age and gender, but not after further adjustment for on admission hsTnT levels [30].
Moreover, assessing microRNA levels post MI is important for studying the relation between temporal changes in specific miRNAs and LV structural remodeling. Profiling miRNAs in the plasma of post-MI patients may hold both mechanistic and prognostic significance. miR-133a levels increased and remain constant till day 90 [31], while other studies showed that miR- 133a and 133b were upregulated directly after the onset of MI and the levels go back to normal at day 5 with similar peak (T0) like that of TnI or reached 4.4 folds increase within 24 hr and back to normal after 1 week [16,21].
Other studies showed that miR-133 is not a good biomarker for AMI diagnosis [32,33]. Among these is a study done by Qing Jing et al which showed that miR-133a was detected with higher levels in plasma from the AMI group, but there were no statistically significant differences in it level among the healthy, non-CHD (coronary heart disease), and CHD groups or miR- 133 levels were mildly but not significantly increased in patients with AMI (n=32)compared with healthy individuals (n=36) [6,32]. This was also detected by Yang et al. who showed no significant differences in miR-133 expression levels among the same groups [33].
To clarify the contradictory results, a diagnostic meta-analysis was done by Baichun Wang et al., in order to estimate the overall accuracy of plasma miR-1, miR-208, and miR-499 as predictive biomarkers of AMI. MiR-208 and miR- 499 showed higher predictive values and there were no significant differences in the predictive values between them, while miR-1 had moderate predictive accuracy [34]. Though many clinical studies aimed to assess the level of these microRNAs in MI patients, contradictory views indicated that further research need to be conducted to describe a comprehensive assessment on the predictive performance.
miR-1 during and after AMI
MiR-1, a muscle-specific miRNA, is known to be expressed highly in cardiac and skeletal muscles with a mild expression in other tissues [35-37]. The miR-1 source in the blood stream of AMI patients is probably exclusively from the heart. The appearance of miR-1 in circulation in AMI patients suggests a release of miR-1 from necrotic myocytes. Thus, it may serve as diagnostic AMI biomarker and this was supported by several studies.
In a study by Ai et al., 93 patients with AMI (age between 30 and 75) were recruited and compared to 66 healthy control subjects. Circulating miR-1 levels in plasma indicated by Ct values was significantly higher in AMI patients than in normal patients with no AMI. Interestingly, as the hospitalized AMI patients were discharged after having received medication for two weeks, the level of circulating miR-1 was restored back to normal value, revealing that miR-1 expression is a potential indicator of AMI [33]. Another group studied the level of miR-1 in 332 patients with suspected ACS that were presented with chest pain with median onset of 3.2 hours before presentation. 106 out of 332 patients diagnosed with ACS showed a higher miR-1 level as compared with the non ACS population. The level of miR-1 was not affected by heparin treatment or platelet inhibition (aspirin, clopidogrel). According to this study, miR-1 showed to be very promising, as its combined diagnostic performance showed to be statistically better than hs-troponin [22].
Furthermore, D'Alessandra et al. evaluated the miRNA expression profile in 17 healthy donors and 33 patients with STsegment elevation MI (STEMI). The results showed that miR-1 level was higher in patients as compared with the control, while miR-122 and -375 were lower than the control. Interestingly, miR-1 level went back to normal baseline level, whereas through day 30, miR-122 remained lower than control. The peak expression of miR-1 as well as Tn1 level happened at a comparable time indicating the importance of miR-1 in detecting cardiac damage [21]. One more study that included 17 patients with AMI compared to 17 healthy individuals with no cardiac disease revealed that circulating miR-1 was upregulated starting 4 hours after AMI symptoms onset, and peaked up after 8 hours [38]. In parallel, a study that included 33 patients with AMI, and 33 non- AMI patients with chest distress and pain within 12 hours of admission compared to healthy volunteers showed by RT-PCR that miR-1 level increased in plasma at 1-3 h, peaked from3-12 h and decreased 12-24 h after coronary artery ligation. MiRNA microarray and real time PCR revealed that miR-1 level was undetectable in 4 healthy individuals. Even though miR-1 was detected with higher level in plasma from the AMI group compared with those from the healthy group, there were no statistically significant difference in its level among healthy, non-CHD (coronary heart disease) and CHD groups [6].
However, Zile aimed to detect changes in miRNA levels post MI in order to assess whether there is a relation between temporal changes in specific miRNAs and left ventricular structural remodeling. 12 patients with confirmed MI and other 12 healthy controls were involved were blood samples were collected after MI (day 2, 5, 28, and 90). Interestingly, on the second day after MI, miR-1 level decreased. On day 5, miR-1 level increased and remained constant till day 9031.
miR-208 and miR-499 in AMI
Zihe Yan et al. focused only on the level of miR-208 as a diagnostic biomarker and showed that its level was significantly higher in AMI group (n=42) immediately after admission compared with the unstable angina UA group (n=22) and healthy controls (n=40), peaking after 12 hours with a positive correlation with serum cTnI level but not with CK-MB [39].
On the other hand, specific investigations on the kinetics of miR-499 in AMI patients have revealed that the relative level of plasma miR-499 in 53 patients with AMI was significantly higher than that in UA group and healthy control group immediately after admission (Th0) peaking at 12 hours and returning to the baseline level after 7 d with no significantly different from that in healthy control group. A positive correlation with cTnI and CK-MB levels was detected which is consistent with their cardiac expression and release from the injured heart [40].
Wagner et al. studied the level of both miR-499 and miR- 208b in comparison with hs-cTnT levels. Blood samples were obtained from a total of 510 patients (113 nSTEMI and 397 STEMI patients) with acute MI with ongoing chest pain for less than 12 h compared with 87 healthy controls. Both miRNAs increased in patients with acute MI as early as 1 hour after the onset of chest pain and remained stable after 3 hours, while they were barely detectable in healthy controls. MiR-208b offered a lower diagnostic accuracy compared with miR-499 and hscTnT [41]. Consistently a study for 32 patients with AMI and 36 patients with atypical chest pain but with no cardiac disease showed that plasma miR-208b and miR-499 levels had a robust elevation and correlated significantly to troponin T and CPK levels, with the highest degree of correlation observed for miR- 49922. miR-499 concentration peaked between 6 h and 12 h in patients with MI [40]. Consistent with other findings, miR-499 level increased at 1-3 h, peaking at 3-12 h and decreasing at 12- 24 h after coronary artery ligation [6].
In parallel Jing et al. indicated that miR-208a displayed higher sensitivity and specificity for diagnosis of AMI compared with the other miRNAs (miR-1,miR-133a and miR-499) by which it was undetectable in plasma from healthy patients and non- AMI patients, also it was absent in non-cardiovascular diseases, (including acute kidney injury, chronic renal failure , stroke and trauma). miR-208a became detectable within 1-4 h of chest pain, when the cTnI level was still detected below the cut-off value. After conventional medical treatment and follow up for 5 patients out of the 33, improvement was associated with decreased levels of these miRNAs and undetectable levels of miR-208a [6]. In contrast, a study with 17 healthy donors and 33 STEMI patients showed that miR-208a was not detectable in healthy control, but it has low level of expression in STEMI patients [21].
Other miRs: miR320 regulates MI
Moreover, other microRNAs were also showed to play a critical role in regulating MI, though they are less studied than the ones mentioned above. One example is miR320 which was shown to play a role in regulating pathophysiological processes including cardiac ischaemia–reperfusion injury [15]. A study done by Ren et al. showed that in murine hearts subjected to ischemia/reperfusion, miR-320 expression was consistently decreased. Furthermore, transgenic mice that exhibited cardiacspecific overexpression of miR-320 showed an increased extent of cardiac apoptosis and infarction size through the inhibition of heat-shock protein [20]. Infarct size was suppressed upon the administration of miR-320 antagomiR. This opens the horizon for using 320 antagomiRs as a treatment to decrease cardiomyocyte loss after MI and reveals a negative regulatory role of miR320 against I/R injury [42,43] . In humans, Jakob et al. showed that upon 1 year follow up in 63 STEMI patients with MACE (cardiac death or recurrent MI), miR-320a levels were highly increased as compared to controls. This suggests that miR-320a stimulates cardiomyocyte death and apoptosis and is involved in pathophysiological mechanisms related for cardiovascular outcome [44].
miRNAs as biomarkers for death prediction
Some studies were interested in using miRNAs for the prediction of cardiac death. Among these a study that involved 873 patients diagnosed with either acute coronary syndrome (ACS) (n=340) or stable angina pectoris (SAP) (n=533). Realtime PCR was used for the assessment of miRNAs (miR-126, miR-223 and miR-197). The aim of this study was to detect if there is a correlation between the level of these 3 miRNAs with cardiovascular death, and thus a prognostic feature. Elevated levels of miR-197 and miR-223 was strongly associated with future cardiovascular death in ACS patients, while miR-126 showed no significant prognostic value [45]. Other study that investigated if there is a relation between circulating miRNAs collected at convalescent stage of AMI and cardiac death prediction in post-AMI patients involved 4160 patients diagnosed with AMI and discharged from hospital, 60 cardiac deaths occurred after discharge. After validation miR-155 and miR-380 showed to be increased in the cardiac death group as compared with the survival group [46].
Others aimed in identifying miRNAs' levels that could be good predictors for cardiac events, by involving Bruneck cohort, a large prospective, population-based study on cardiovascular disease. In 1995 the study involved 820 participants of Caucasian origin, out of these 47 participants experienced MI over the 10-year follow-up period (an incidence rate of 5.3 per 1,000 person-years). Cox regression analysis showed that miR- 126, miR-197, and miR-223 have the strongest associations with incident MI. MiR-126 was positively associated with incident MI, whereas miR-197and miR-223 were inversely related to disease risk [47].
Conclusion
Currently none of the listed miRs are used in clinical diagnosis, despite the promising results gained from different research studies and this is mainly due to the failure to beat cost and time effectively the widely used cTnI test. Despite the contradictory results implicating miRNAs as biomarkers in MI diagnosis, treatment, and prognosis, the technical ease with which these circulating miRs are detected make them the best hits for pharma and biotech industries. Additional clinical trials are needed however in different ethnicities before any official approval for the use of a given miR as a biomarker. More importantly technical hurdles related to standardization of protocols, starting from sample collection and ending with expression quantitation should be overcome in order to use these promising molecules in the clinic.
References
- Ono K, KuwabaraY, Han J. MicroRNAs and cardiovascular diseases. FEBS J. 2011;278(10):1619-33.
- Institute of Medicine. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. 2010.
- Pires-Gomes A, Pérez-PomaresJ. The Epicardium and Coronary Artery Formation. J Dev Biol. 2013;1:186-202.
- Zhu H, Fan GC. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc Res. 2012;94(2):284-92.
- Mendis S, Thygesen K, Kuulasmaa K, et al. World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. 2011;40(1):139-46.
- Wang GK, Zhu JQ, Zhang JT, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31(6):659-66.
- Jaffe AS, Ravkilde J, Roberts R, et al. It’s Time for a Change to a Troponin Standard. Circulation. 2000;102:1216-20.
- Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. ProcNatlAcadSci USA. 2011;108(12):5003-08.
- Creemers EE, Tijsen AJ, Pinto YM. Circulating MicroRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110(3):483-95.
- Gupta SK, Bang C, Thum T. Circulating MicroRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. CircCardiovasc Genet. 2010;3(5):484-88.
- Ambros V. MicroRNA Pathways in Flies and Worms. Cell. 2003;113(6):673-76.
- Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423-33.
- Chen TS, Lai RC, Lee MM, et al. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2009;38(1):215-24.
- Kosaka N, Iguchi H, Yoshioka Y, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442-52.
- Mcmanus DD, Freedman JE. MicroRNAs in platelet function and cardiovascular disease. Nat Rev Cardiol. 2015;12(12):1-7.
- Wang R, Li N, Zhang Y, et al. Circulating MicroRNAs are Promising Novel Biomarkers of Acute Myocardial Infarction. Intern Med. 2011;50(17):1789-95.
- Condorelli G, Latronico MVG, Dorn GW. MicroRNAs in heart disease: Putative novel therapeutic targets? Eur Heart J. 2010;31(6):649-58.
- De Rosa S, Fichtlscherer S, Lehmann R, et al. Transcoronary concentration gradients of circulating MicroRNAs. Circulation. 2011;124(18):1936-44.
- Schulte C, Zeller T. MicroRNA-based diagnostics and therapy in cardiovascular disease-Summing up the facts. CardiovascDiagnTher. 2015;5(1):17-36.
- Hu Y, Matkovich SJ, Hecker PA, et al. Epitranscriptional orchestration of genetic reprogramming is an emergent property of stress-regulated cardiac microRNAs. ProcNatlAcadSci USA. 2012;109(48):19864-69.
- D’Alessandra Y, Devanna P, Limana F, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31(22):2765-73.
- Corsten MF, Dennert R, Jochems S, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. CircCardiovasc Genet. 2010;3(6):499-506.
- Ali SS, Kala C, Abid M, et al. Pathological microRNAs in acute cardiovascular diseases and microRNA therapeutics. J Acute Dis. 2015;5(1):9-15.
- French KJ, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525-38.
- Li C, Fang Z, Jiang T, et al. Serum microRNAs profile from genome-wide serves as a fingerprint for diagnosis of acute myocardial infarction and angina pectoris. BMC Med Genomics. 2013;6:16.
- Vogel B, Keller A, Frese KS, et al. Refining diagnostic MicroRNA signatures by whole-miRNome kinetic analysis in acute myocardial infarction. Clin Chem. 2013;59(2):410-18.
- Liu J, Hao DD, Zhang JS, et al. Hydrogen sulphide inhibits cardiomyocyte hypertrophy by up-regulating miR-133a. BiochemBiophys Res Commun. 2011;413(2):342-47.
- Wang F, Long G, Zhao C, et al. Plasma microRNA-133a is a new marker for both acute myocardial infarction and underlying coronary artery stenosis. J Transl Med. 2013;11(1):222.
- Eitel I, Adams V, Dieterich P, et al. Relation of circulating MicroRNA-133a concentrations with myocardial damage and clinical prognosis in ST-elevation myocardial infarction. Am Heart J. 2012;164(5):706-14.
- Widera C, Gupta SK, Lorenzen JM, et al. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol. 2011;51(5):872-75.
- Zile MR, Mehurg SM, Arroyo JE, et al. Relationship between the temporal profile of plasma microRNA and left ventricular remodeling in patients after myocardial infarction. CircCardiovasc Genet. 2011;4(6):614-19.
- Adachi T, Nakanishi M, Otsuka Y, et al. Brief Communications. Clin Chem. 2010;56(7):1183-1185.
- Ai J, Zhang R, Li Y, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. BiochemBiophys Res Commun. 2010;391(1):73-77.
- Liu X, Fan Z, Zhao T, et al. Plasma miR-1, miR-208, miR-499 as potential predictive biomarkers for acute myocardial infarction: An independent study of Han population. ExpGerontol. 2015;72:230-38.
- Xiao L, Xiao J, Luo X, et al. Feedback remodeling of cardiac potassium current expression: A novel potential mechanism for control of repolarization reserve. Circulation. 2008;118(10):983-92.
- Luo X. Mitogen Activated Protein Kinase-Dependent Activation of c-Jun and c-Fos is required for Neuronal differentiation but not for Growth and Stress Reposne in PC12 cells. J Cell Physiol. 2006;207(1):12-22.
- Terentyev D, Belevych AE, Terentyeva R, et al. MiR-1 overexpression enhances ca2+ release and promotes cardiac arrhythmogenesis by targeting pp2a regulatory subunit b56α and causing camkii-dependent hyperphosphorylation of RyR2. Circ Res. 2009;104(4):514-21.
- Long G, Wang F, Duan Q, et al. Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction.Int J Biol Sci. 2012;8(6):811-18.
- Han Z, Zhang L, Yuan L, et al. Change of plasma microRNA-208 level in acute myocardial infarction patients and its clinical significance. Ann Transl Med. 2015;3(20):307.
- Chen X, Zhang L, Su T, et al. Kinetics of plasma microRNA-499 expression in acute myocardial infarction. J Thorac Dis. 2015;7(5):890-96.
- Devaux Y, Vausort M, Goretti E, et al. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin Chem. 2012;58(3):559-67.
- Ren X, Wu J, Wang X, et al. NIH Public Access. Injury. 2009;119(17):2357-66.
- Small EM, Frost RJA, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121(8):1022-32.
- Jakob P, Kacprowski T, Briand-Schumacher S, et al. Profiling and validation of circulating microRNAs for cardiovascular events in patients presenting with ST-segment elevation myocardial infarction. Eur Heart J. 2017;38(7):511-15.
- Schulte C, Molz S, Appelbaum S, et al. MiRNA-197 and miRNA-223 predict cardiovascular death in a cohort of patients with symptomatic coronary artery disease. PLoS One. 2015;10(12):1-12.
- Matsumoto S, Sakata Y, Nakatani D, et al. A subset of circulating microRNAs are predictive for cardiac death after discharge for acute myocardial infarction. BiochemBiophys Res Commun. 2012;427(2):280-84.
- Zampetaki A, Willeit P, Tilling L, et al. Prospective study on circulating microRNAs and risk of myocardial infarction. J Am CollCardiol. 2012;60(4):290-99.