- Biomedical Research (2014) Volume 25, Issue 4
Microbial contaminants in the herbal preparation from green pawpaw fruits.
Amosu AM1*, Degun AM2 and Esan EB31Department of Public Health, School of Health Sciences, University of Venda, P/Bag X5050, Thohoyandou 0950, South Africa
2Faculty of Public Health, Department of Community Medicine, University College Hospital, Ibadan, Nigeria
3Department of Chemical & Environmental Sciences, Babcock University, Ilisan-Remo, Nigeria
- *Corresponding Author:
- A.M. Amosu
Department of Public Health
University of Venda
PMB X5050, Thohoyandou 0950, South Africa
Accepted date: July 07 2014
Abstract
This study is designed to investigate the presence of diverse micro-organisms in the herbal preparations made from green, unripe mature pawpaw fruits for the treatment of stomach ulcer. Samples of pawpaw fruits were collected from a pawpaw plantation in Ibadan, Nigeria, and used within one hour of being harvested. Pour plate method was employed for the microbiological and biomedical charaterisation of isolates. The results confirmed the presence of yeast and pathogenic organisms including bacteria and fungi. Among them are Alcalogenes faecalis, M. kristianae, B. polymyxa, M. varians, Corynebacterium spp. as well as A. iwoffi; which is the most pathogenic bacteria among the isolates, as the organism is known to cause food spoilage and lower respiratory tract infection (LRTI) in humans. This herbal preparation poses a potential hazard to human health if not consumed fresh. A suitable means of preservation such as cold storage is therefore required if the preparation is to be stored. In the alternative, the use of fresh preparations on a daily basis is highly recommended.
Keywords
Herbal preparations, pawpaw fruits, stomach ulcer, lower respiratory tract infection
Introduction
The medicinal benefits of herbs have been known for centuries. The application of herbal drugs for the cure of a good number of ailments is now a common occurrence in many communities, most especially among the rural populace, probably due to their low socio-economic status and the high cost of orthodox medicines. Several herbalists, also known as Trado-medical practitioners have made claims on the therapeutic effects of preparations from virtually all parts of the pawpaw plant, Carica papaya, and especially the unripe fruit, without due reference to the potential dangers associated with the preparations especially when not consumed fresh.
In Nigeria, especially in the rural areas, herbal medicines are crude preparations of various kinds of medicinal plants, involving a dried plant or any of its parts such as the leaf, stem, root, flower, fruit or seed. Many of them are believed to have medicinal properties and are used to treat minor illnesses and disturbances. They are usually promoted as natural and safe and to be the preferred choice to western medicines. As natural products, bacteria, fungi and especially molds, may degrade all parts of the plants. The non- scientific methods of cultivation and collection, inappropriate harvesting and cleaning, unsuitable transportation, prolonged drying and storage, poor hygiene of producers and prevailing climatic conditions render the raw plant materials prone to infestations and exposure to many microbial contaminants.
Raw materials are most often degraded by microorganisms before harvesting, during handling and after prolonged storage [1,2]. The presence of large numbers of microbes can be harmful for human consumption. The risk of mycotoxin production, especially aflatoxin, should be considered in the process of the herbal preparation because of the proven mutagenic, carcinogenic, teratogenic, neurotoxic, nephrotoxic and immunosuppressive activities of the fungus [3-6].
The pawpaw is a unique fruit crop with high nutritional value and potential for both fresh and processed market uses. As a food source, pawpaw exceeds apple, peach, and grapes in vitamins, minerals, amino acid, and food energy values. The green fruit is reported to contain protein, fat, carbohydrate, fiber, ash, Ca, P, Fe, Na, K, betacarotene equivalent, thiamine, riboflavin, niacin, ascorbic acid and Vitamin E [7]. It has also been found to contain sinigrin, the enzyme myrosin, and carpasemine [8].
There are valuable natural compounds in the plant, which have anti-carcinogenic properties. An alkaloid, asimicin, is found in the seeds, leaves, and bark of pawpaw and is reported to be a pesticide. Aromatic compounds in the fruit have potential for use in cosmetics and home products [9]. Research has also shown that the pawpaw plant has a diversity of natural compounds in fruit, leaves, bark, and twigs. Annoaceous acetogenins, a class of compounds, occurs in the leaves and twigs and has been reported to possess anti-tumor properties. It is commonly known for its food and nutritional values throughout the world. The leaves, fruits and latex obtained from the papaya plant are used medicinally and for various purposes [9]. The fruit has been found to contain certain immunestimulating and anti-oxidant agents [10]. Immature fruits and roots are used for their abortifacient activity [11,12]. The seeds are used as a potential post-testicular antifertility drug [13-15], while the pulp is used by African hospitals for treating wounds and burns [16].
The latex and the seeds are used in the treatment of gastrointestinal nematode infections and they have shown anthelmintic activity [17]. The seeds and immature fruit have shown inhibitory activity against human enteric pathogens [9,18,19]. Pawpaw has many biologically active compounds including chymopapain and papain, which are believed to aid digestion. Papain is used for treating arthritis while the softening property it possesses is employed in the treatment of chronic forms of scaly eczema, cutaneous tubercles, corns, warts and other skin affections resulting from irritation [9].
In one study conducted in Dar es Salam, it was observed that liquid and powdered herbal medicines prepared locally by herbalists had high levels of bacterial contamination [20]. However, water, acetone and ethanol extracts of papaya leaves showed no microbial activity [21-23].
This study was carried out to examine the purity and hence the safety of the herbal preparation of unripe pawpaw fruit for the cure of stomach ulcer, as claimed by several herbalists or Trado-medical practitioners in Nigeria.
Materials and Methods
Herbal preparation for the treatment of stomach ulcer
In the herbal treatment of stomach ulcer by the local herbalists, a big unripe pawpaw fruit is cut into pieces. The peel or seeds are not removed. The whole fruit is simply cut into cubes, and then soaked in a bowl containing 1200mls of water for four days. The resulting mixture is sieved and half a glass of the liquid is prescribed for the stomach ulcer patient to be taken three times daily for two weeks. This has been claimed to be a very good remedy for the treatment of stomach ulcer [24].
For this study, samples of pawpaw fruits were collected from a pawpaw plantation in Ibadan, Nigeria, and used within the period of one hour of being harvested.
Reagents for examination
Ethanol (95%) and distilled water.
--- Nutrient Agar used as a general purpose agar for the culture of non-fastidious organisms.
----Potato dextrose Agar (PDA), a selective medium for the isolation of yeasts and fungi.
----Deoxycholate Citrate Agar, a pink powder; which is a selective medium for the isolation of Shigella and Salmonella spp.
Procedure
1200mls of distilled water was measured into two bowls. Four green and mature unripe pawpaw fruits were weighed, washed with detergent, rinsed with distilled water and left to dry. The pawpaw fruits were sterilized with ethanol (95%), sliced into cubes with a sterile knife and then placed in the bowls containing distilled water, and allowed to stand for 1, 2, 3, 4, 8 days. Samples of the resulting solution were collected and sieved on the fourth day after standing [24].
Sterilization of materials used
All the glassware and media used were sterilized by autoclaving. The media were prepared in a conical flask plugged with cotton wool and wrapped with aluminum foil before autoclaving. Sterilization in the autoclave was carried out at 121°C for 15 minutes.
Isolation
1.0g of the sieved pawpaw solution was dissolved in 10mls of distilled water and serial dilution carried out in a dilution of 1015. One milliliter of the fourteenth dilution was poured aseptically in plates of molten nutrient agar cooled to about 43°C, and then immediately swirled gently for about ten seconds before setting. The same procedure was repeated for every sample at the predetermined isolation time.
Microbiological and biochemical analysis
In order to identify bacterial species, the microbiological techniques employed included inoculation, Gram staining, colony and morphological characterisation for analyses of physical and structural features of organisms. Biochemical tests carried out included catalase, oxidase citrate, indole urease test, methyl red, and sugar fermentation tests. Identification of the fungi species was carried out using Potato Dextrose Agar (PDA), and was based on colonial morphology (pigmentation, mycelia texture, growth pattern and colour) and microscopy which included hyphae structure and asexual spore appearance (type, colour, shape and texture) [25].
Characterization and identification of organisms
The mixed microbial culture isolates from nutrient agar were streaked on fresh nutrient agar aseptically, to obtain pure cultures of the micro-organisms. The stock cultures were prepared for the slants by inoculation unto nutrient agar slants. These slants were incubated at30°C for 24 hours, and were then stored in the refrigerator. Pure cultures of fungal isolates were also made by inoculation unto PDA slants. These were incubated at 30°C for 48 hours after which they were stored in the refrigerator.
Identification of bacterial isolates
Pure cultures of the isolates were subjected to various biochemical tests to determine the probable identity of the bacteria. The result of each test was recorded and the probable identity of the isolate was determined by the use of Bergey's manual of determinative bacteriology [26]. The following biochemical tests were carried out:
Gram stain
Smears of 24 hour culture were made on clean greasefree slides and heat fixed. The slides were immersed in crystal violet solution and rinsed gently with distilled water. The smear was then flooded with Gram’s iodine for a minute, accompanied by an immediate rinsing with distilled water and subsequent decolourization by flooding with 95% ethanol. The slides were rinsed again, counterstained with safranin for a minute, washed off with distilled water and allowed to dry after which a drop of cedar wood immersion oil was applied, and observed under the immersion objective of the compound light microscope [27].
Spore staining
Spore staining technique was used to detect the presence of spores. Smears of 48 hours cultures of isolates were heat fixed on different glass slides. These were flooded with malachite green stain and heated over a beaker of boiling water for l0 minutes. More of the malachite green stain was continually added to the slides to prevent drying. The slides were then washed and counter stained with safranin for 20 seconds, washed again, blot dried and examined under oil immersion lens. While the vegetative portion of the organism stained pink to red, the spores stained green [28].
Motility test
This test was carried out using Edwards and Wing motility test medium. The semi solid medium was inoculated with different bacterial isolates by stabbing with a sterile inoculating needle at the centre of the medium column to over half the depth. The mobile organisms grew and spread out from the line of puncture while the nonmotile organisms grew along the line of puncture [28].
Oxidase Test
In order to detect the presence of cytochrome oxidase enzyme, which occurs in some bacteria that carry out respiration where oxygen is the terminal electron acceptor, a piece of No.1 Whatrnan’s filter paper was impregnated with oxidase reagent (tetramethyl-pphenylenediamine) in a Petri dish. A sterile glass rod was used to smear some bacterial growth from the 24 hour culture onto the impregnated filter paper. The production of purple colouration within five to ten seconds indicated the presence of oxidase while a delayed reaction was regarded as negative [29].
Other tests carried out included Catalase test to detect the presence or absence of catalase enzyme in each of the bacterial isolates, which catalyses the breakdown of hydrogen peroxide to release gaseous hydrogen and water (which otherwise can damage the enzymes), nucleic acids, and small molecules in the cell due to its highly reactive nature; Indole test to determine the bacteria producing the enzyme tryptophanase which hydrolyzes tryptophan to pyruvic acid, ammonia and indole; Urease test to determine if the test organism was capable of producing urease, an enzyme that splits urea molecule into carbon dioxide and ammonia; Citrate test to see if the test organism was capable of utilizing citrate as sole carbon source for metabolism with resulting alkalinity; Methyl Red test to show if the test organisms carried out mixed fermentation and Voges-Proskauer (VP) test to determine if the test organism produced acetyl methyl carbinol from glucose, which will be a positive result for the VP test [30].
Identification of fungal isolates
Pure cultures of fungi isolates were established using single sporing method [30]. Identification was based on colonial morphology (colour, mycelia texture, growth pattern and reverse plate pigmentation) and microscopy, which included hyphae structure and spore appearance (type, colour, and shape) [25]. Pure cultures of identified species were carefully labeled and maintained on Potato dextrose agar at 4”C.
Starch hydrolysis Test
This test was carried out to determine the ability of the isolates to hydrolyze starch by the production of enzymes such as alpha-amylase and oligo-1, 6-glucosidase.
Results
Several bacterial isolates were identified and characterized during the isolation from the pawpaw solution as shown in as under.
Microbial contaminants in herbal preparation from pawpaw
+ = Positive; - = Negative
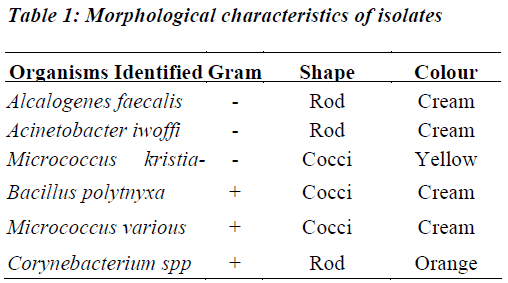
Table 1 shows organisms; which were classified according to their morphological structures. Alcalogenes feacalis is gram negative, rod shaped and cream in colour. Acinetobacter iwoffi is gram negative, rod shaped and also cream in colour. Micrococcus kristianae are gram negative cocci, and yellow in colour. Bacillus polymyxa are gram positive cocci and cream coloured. Micrococcus varians is gram positive, cocci shaped and also cream coloured. Corynebacterium spp is gram positive, rod shaped and yellow in colour.
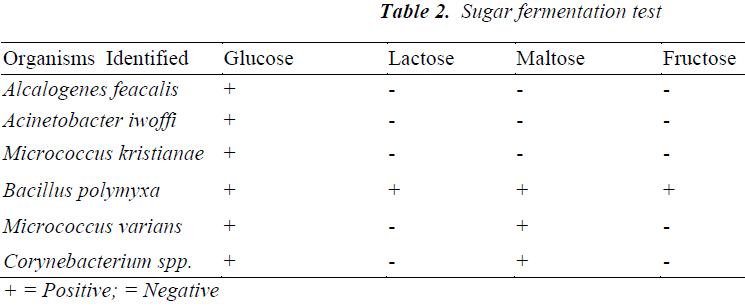
Table 2 contains organisms; which were classified according to fermentation of sugars.
Alcalogenes feacalis, Acinetobacter iwoffi and Micrococcus kristianae fermented glucose but did not ferment lactose, maltose, and fructose.
Bacillus polymyxa fermented Glucose, lactose, maltose, and also ferments fructose.
Micrococcus varians and Corynebacterium spp. fermented Glucose and maltose, but did not ferment lactose, and fructose.
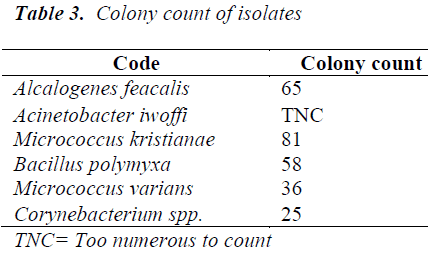
Table 3 shows colony count of the various organisms cultured from the herbal preparation, where Acinetobacter iwoffi happened to be present in large numbers.
+ = Positive; - = Negative
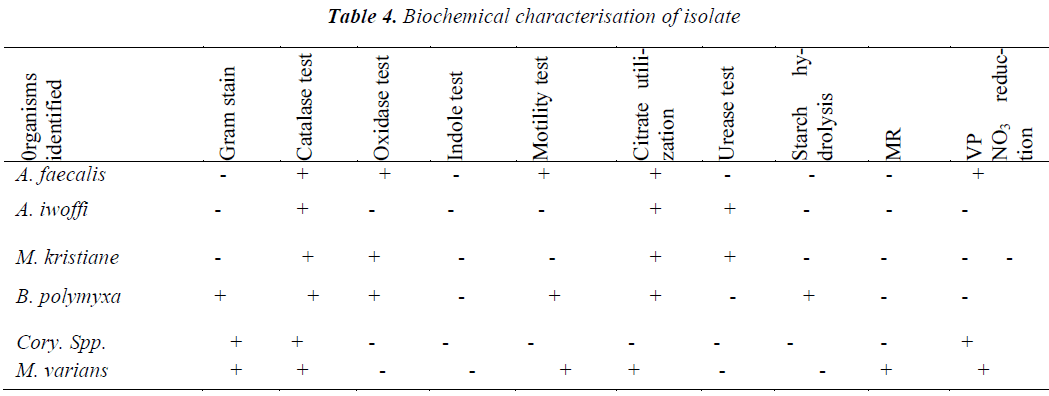
Table 4 shows the biochemical characteristics of the micro-organisms isolated.
Discussion
This study investigated microorganisms associated with the herbal preparation from unripe pawpaw (Carica papaya) fruit being employed by herbalists for the cure of stomach ulcer. Findings from the study demonstrated the presence of pathogenic bacteria in the preparation, despite the sterile procedures employed; which is unlikely to be found in most herbal homes. Among the organisms cultured, Alcalogenes feacalis is a mammalian parasite that multiplies in the respiratory epithelial cells, Acinetobacter iwoffi causes food spoilage, is parasitic, and also causes respiratory tract infections in human, Micrococcus kristianae is non-pathogenic, widely spread in soil and water and is also a normal flora of the skin, Corynebacterium spp. is an air borne pathogen; which mostly causes respiratory tract infections. This shows that when a rich fruit like pawpaw is exposed to high levels of moisture and water vapour, whether sterilized or not, microorganisms of different types thrive very well [7].
The observations in this study corroborate those of previous investigations, where it was observed that almost all the collected herbal samples were contaminated with molds and aerobic mesophilic bacteria [31]. The most likely sources of contaminants in herbal preparations might be the unhygienic conditions of most herbal homes, use of dirty utensils, polluted water and exposure to dust and other forms of particulate matter.
Conclusion
The pawpaw fruit has numerous beneficial effects and all parts of the pawpaw tree (stem, root, leaf, fruit, seeds, and flower) have been found to be useful. There is however, an urgent need for practitioners especially those in the villages, to be educated on the hygienic preparation, storage and dispensing of herbal medicines to prevent microbial contamination. Adequate efforts should be made for the control of pathogenic organisms in the herbal preparations, so as to ensure their safety for human consumption.
References
- Mathe A. Storage of Medicinal and Aromatic Plants: An Important Item of Good Agricultural and Good Manufacturing Practice Workshop on Storage of Medicinal Plants By Society of Medicinal Plant. Saale1995; 5-19.
- Kenneth C. The Herb, Spice and Medicinal Plant. Digest 1989; 7: 1-5.
- Refai K. Aflatoxin and Aflatoxicosis. Journal Egyptian Veterinary Medical Association 1988; 48(1): 1–19.
- Scimeca JA. Naturally occurring orally active dietary carcinogens: In: handbook of Human toxicology. Massaro E.J. CRC Press 1995; 435-437.
- F.A.O. Food safety and quality as affected by animal feed- stuff. Twenty second F.A.O Regional Conference for Europe, Portugal 2000.
- Höhler D. A brief survey on important mycotoxin and possible detoxification methods Feed Technology 2000; 4 (5/6): 44-46.
- Gaufberg, SM. Threatened Abortion. eMedicine. Eds. Roy Alson, et al. 17 Dec. 2008. Medscape 31 Aug. 2009 <http://emedicine.medscape.com/article/795439-overview>.
- Okwu DE, Okwu E. Chemical compositions of Spondias mombin linn. Plant parts. Journal of Sustainable Agricultural Environment 2004; (6):140-147.
- Krishna KL, Paridhavi M, Jagruti AP. Review on nutritional, medicinal and pharmacological properties of Papaya (Carica papaya). Natural Pro Radiance 2008; 7: 364-373
- Aruoma OI, Colognato R, Fontana I, Gartlon J, Migliore L, Koike, Coecke S,Lamy E, Mersch-Sundermann V, Laurenza I, Benzi L, Yoshino, Kobayashi K, Lee MC. Molecular effects of fermented papaya preparation on oxidative damage, MAP Kinase activation and modulation of the benzo[a]pyrene mediated Genotoxicity. Biofactors 2006; (26): 147-159.
- Cherian T. Effect of papaya latex extract on gravid and non-gravid rat uterine Preparations in-vitro. Journal of Ethno Pharmacology 2006; (70): 205-212.
- Sharma HN, Mahanta HC. Modulation of morphological changes of endometrial Surface epithelium by administration of composite root extract in albino rat. Contraception 2000; 62: 51-54.
- Lohiya NK, Pathak N, Mishra PK, Maniyannan B. Contraceptive evaluation and Toxicological study of aqueous extract of the seeds of Carica papaya in male Rabbits. Journal of Ethno pharmacology 2000; (70): 17-27.
- Lohiya NK, Mishra PK, Pathak N, Manivannan B, Bhande SS, Panneerdoss S, Sriram S. Efficacy trial on the purified compounds of the seeds of Carica papaya for male contraception in albino rat. Reproductive Toxicology 2005; (20): 135-148.
- Lohiya NK, Manivannan B, Garg S. Toxicological investigations on the Methanol sub-fraction of the seeds of Carica papaya as a male contraceptive in albino rats. Reproductive Toxicology 2006; (22): 461-468.
- Starley IF, Mohammed P, Schneider G, Bickler SW. The treatment of paediatrics burns using topical papaya. Burns 1999; 25:636-639.
- Stepek G, Buttle DJ, Duce IR, Lowe A, Behnke JM. Assessment of anthelmintic Effect of natural plant cysteine proteases against the gastrointestinal nematode, Heligmosomoidespolygyrus, in-vitro. Parasitology 2005; 130: 203-211.
- Osato JA, Santiago LA, Remo GM, Cuadra MS, Mori A. Antimicrobial and Antioxidant activities of unripe papaya. Life Science 1993; (53): 1383-1389.
- Afolayan AJ. Extracts from the shoots of Arctotis arctotoides inhibit the growth of bacteria and fungi. Pharmaceutical Biology 2003; (4): 22-25.
- Justin-Temu M, Lyamuya EF, Makwaya C, Antony PR, Mloka D. Microbes in herbal drugs. African Journal of Health Sciences. 1998; (5): 140-143.
- Nkuo-Akenji T, Ndip R, McThomas A, Fru EC. Anti Salmonella activity of medicinal plants from Cameroon. Central African Journal of Medicine 2001; (47):153-158.
- Dawkins G, Hewitt H, W’Int Y, Obiefuna PC, W’Int B. Antibacterial effects of Carica papaya fruit on common wound organisms. West Indian Medical Journal 2003; (52): 290-292.
- Nayak SB, Pinto PL, Maharaj D. Wound healing activity of Carica papaya in experimentally induced diabetic rats. Indian Journal of Experimental Biology 2007; (45): 739-743.
- Addo A. Nature power, Generation press Ltd, 3rd edition. 2006; 57-62.
- Jansen PH. Identifying the Dominant Soil Bacterial Taxa in Libraries of 16S rRNA and 16S rRNA Genes. Applied Environmental Microbiology 2006; 72 (3): 1719-1728.
- Forbes B; Daniel A; Sahm F; Alice A & Weiss-feld S. Chapter 9 in Bailey and Scott’s Diagnostic Microbiology, 11th Ed. Mosby-Yearbook, Inc., St. Louis, MO; 2002.
- Norris JR, Helen Swain. Chapter II in Methods in Microbiology, Volume 1, SA, edited by Norris, J. R & Ribbons, D.W. Academic Press, Ltd London; 1971.
- Leboffe MJ, Pierce BE. Microbiology: laboratory theory and applications. Morton Publishing Company, Englewood CO; 2002.
- McDonald WL, Jamaludin R, Mackereth G, Hansen M. Characterization of a Brucella sp. strain as a marinemammal type despite isolation from a patient with Spinal osteomyelitis in New Zealand. Journal of Clinical Microbiology 2006; (44): 4363-4370.
- Leslie JF, Summerell BA. The Fusarium Laboratory Manual. Blackwell Publishing Professional, Ames, IA, USA; 2006.
- Stojadinov J. Ispitivanje mikrobiolške kontaminacije pitome nane (Mentha piperita L). Matieres medicales 1998; (XVII): 45-53.