Research Article - Current Pediatric Research (2017) Volume 21, Issue 2
MFSD8 mutation causing variant late infantile neuronal ceroid lipofuscinosis (vLINCL) in three Palestinian siblings of Arab Descent.
Amal Y Kentab*
Division of Pediatric Neurology, Department of Pediatrics, College of Medicine and King Khalid University Hospital, King Saud University, Riyadh, Saudi Arabia.
- Corresponding Author:
- Amal Y Kentab
Assistant Professor, Division of Pediatric Neurology
Department of Pediatrics, College of Medicine and King Khalid University Hospital
King Saud University, P.O. Box 2925, Riyadh 11461, Saudi Arabia
Tel: 966553400308
Fax: +966(1) 469-1512
E-mail: amkentab@hotmail.com; akentab@ksu.edu.sa
Accepted date: February 22, 2017
Abstract
The Neuronal Ceroid-Lipofuscinoses (NCL) is a group of rare neurodegenerative disorders characterized by an accumulation of autofluorescent lipopigments in neurons and extraneuronal tissues. Clinical manifestations include seizures, progressive mental and motor deterioration, myoclonus, visual failure, dementia and premature death. Classically, NCL-affected individuals have been classified into six categories, based on the clinical onset of symptoms. Fourteen genetic forms of NCL (CLN1 to CLN14) have been described to date. The variant late-infantile form of the disease has been linked to CLN5, CLN6, CLN7 (MFSD8) and CLN8 mutations. The author reports the clinical and neuroradiological features and molecular mutational analysis of 3 Palestinian siblings (two females, one male), products of a consanguineous marriage and in vitro fertilization, who were affected by the variant late-infantile NCL due to a homozygous mutation of CLN7 (MFSD8) gene. The author highlights the importance of an early recognition of this phenotype and an early confirmation of molecular diagnosis, which will allow for early and proper genetic counseling, including pre-implantation genetic diagnosis (PGD) and future family planning.
Keywords
Neuronal ceroid lipofuscinosis, Neurodegenerative disorders, CLN7, MFSD8, Myoclonic epilepsy, Visual failure.
Introduction
The neuronal ceroid-lipofuscinoses (NCL or CLN), also known as Batten Disease, are a clinically and genetically heterogeneous group of lysosomal storage neurodegenerative disorders which are characterized by the accumulation of abnormal autofluorescent lipopigmentpositive materials in the cytoplasm of neurons, skin and skeletal muscle [1-4]. It is one of the most frequently inherited childhood-onset neurodegenerative diseases. Its prevalence ranges from 1:100,000 to 1:1,000,000 live births worldwide. Most NCLs are inherited in a recessive manner and are clinically characterized by a variable age at onset, progressive mental and motor deterioration, seizures, myoclonus, dementia, visual impairment and premature death [1]. Classically, NCL-affected individuals have been classified into six categories (congenital, infantile, late infantile, variant late infantile, juvenile and adult), based on the clinical onset of symptoms, however, some patients cannot be easily included in a specific group because of overlapping of clinical and genetic features [5]. Fourteen NCL genetic forms (CLN1 to CLN14) have been described to date that share common clinical and pathological etiologies [2,6]. More than 360 NCL aetiological mutations have been reported, most of which have been included in the NCL Mutation Database (http://ucl.ac.uk/ncl/mutation) [7].
To date, the variant late infantile form of the disease (vLINCL) is the most genetically heterogeneous subtype as it has been linked to mutations in at least six genes, namely CLN5, CLN6, CLN7 and CLN8 [2,3]. Homozygous or compound heterozygous mutations in the MFSD8 gene (MIM 611124) were previously reported to underlie the vLINCL termed CLN7 disease (MIM 610951) [8-12].
MFSD8/CLN7 gene encodes for CLN7, a putative lysosomal transporter (major facilitator superfamily domain containing protein 12) protein on chromosome 4q28.1–q28.2, localized to the lysosomal membrane and belongs to the major facilitator superfamily (MFS) [12]. Although this protein is ubiquitously expressed, high transcript concentrations have been identified in specific brain locations, such as the cerebellar cortex and the hippocampus [13]. More than 30 pathogenic sequence variants have been described so far in MFSD8, most being homozygous missense mutations (http://ucl.ac.uk/ncl/mutation) [2]. It has been proposed that this kind of mutations do not affect protein subcellular location, but might produce disturbances regarding their functional properties [7]. Almost all MFSD8 mutations are isolated and some founder effects have been described [10].
Despite advances in the diagnosis of neurodegenerative disorders, CLN7 disease remains clinically difficult to distinguish from other forms of late-infantile lipofuscinosis. This article provides further information regarding the CLN7 phenotype in Arab populations as the author describes the characteristic features of vLINCL in 3 siblings from a Palestine inbred family with a (c.103 C>T, p.Arg35X) homozygous pathogenic mutation of the MFSD8 gene confirmed by genome-wide homozygosity mapping and candidate gene direct sequencing aiming to enhance an awareness about this disease for early recognition, diagnosis, genetic counseling, and future family planning.
Patients and Methods
The subjects of this study were three children (siblings), two females and one male originating from an Arab Palestinian consanguineous family with vLINCL, who were diagnosed and followed up at the outpatient clinic of the King Khalid University Hospital Riyadh, Division of pediatric Neurology.
Data about age, sex, consanguinity, family history, clinical features (motor deterioration, ataxia, epilepsy, mental deterioration, truncal rigidity, limb spasticity, tremors, visual failure and speech impairment), investigations (Complete Blood Count (CBC), liver and renal profile , basic metabolic work-up (venous blood gas, serum lactate, ammonia, phytanic acid, Very Long Chain Phytanic Acid (VLCFA), serum amino acid chromatography, acylcarnitines, urine organic acids), standard karyotyping, vaculated lymphocytes, skin biopsy, muscle biopsy, electroencephalogram (EEG), Electroretinogram (ERG), visual evoked potential (VEP), brain auditory evoked potential (BAEP), brain CT scan, brain MRI and ophthalmological evaluation), and the outcome were collected from the patients record. DNA was extracted from EDTA-blood samples obtained from affected children and their parents after obtaining parents written informed consent. Molecular analysis by genome-wide homozygosity mapping was performed, followed by candidate gene direct sequencing of the 12 coding exons (2e13) of the MFSD8 gene (gene MIM; 611124).
Case Report 1
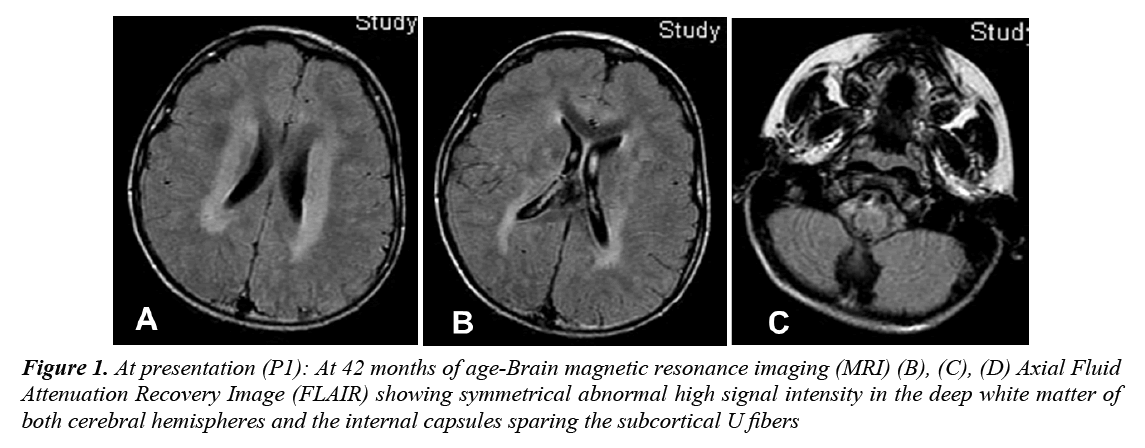
A 4 year old Palestinian girl (Arab ethnicity) presented to King Khalid University Hospital (KKUH), Riyadh, with psychomotor regression and frequent drop attacks, which began at the age of 3. She was a product of full-term In Vitro Fertilization (IVF) pregnancy after 17 years of primary infertility, delivered by normal spontaneous vaginal delivery with uneventful perinatal and natal periods. Her developmental status was mildly delayed: sat up without support at 9 months, walked at 19 months and developed few words with inability to say a complete sentence by 24 months. She began to lose motor milestones at 3 years with the appearance of irritability, sleeplessness, clumsiness, unsteadiness of gait and occasional drop attacks with subsequent trauma to the head. She was evaluated at a local hospital and was found to have spastic lower limbs, optic atrophy and left ulnar fracture. Brain MRI showed periventricular white matter changes (Figure 1). The patient continued to regress; the drop attacks increased in severity and frequency. At the age of 3 years and 6 months, she developed variable seizures including atypical absence seizures, Generalized Tonic-Clonic (GTC) seizures, and recurrent myoclonic jerks. Later on, she developed focal seizures with secondary generalization, followed by progressive ataxia and regression of speech. Within 6 months she became increasingly ataxic, incontinent and she lost her walking ability as well as vocalization. The parents, who are first cousins, have two far relatives with seizures and developmental delay. At presentation, she was confined to a wheelchair, irritable, normocephalic with no dysmorphism. She unable to fixate or follow objects with her eyes and had profound impairment of speech. She had psychomotor deterioration with loss of ability to stand or sit independently, truncal ataxia, intermittent myoclonic jerks, spastic quadriplegia and difficulty in swallowing with significant drooling. Fundus examination showed pallor of the optic disc with no retinal abnormalities. Systemic examination was normal, and there was no organomegaly.
Figure 1: At presentation (P1): At 42 months of age-Brain magnetic resonance imaging (MRI) (B), (C), (D) Axial Fluid Attenuation Recovery Image (FLAIR) showing symmetrical abnormal high signal intensity in the deep white matter of both cerebral hemispheres and the internal capsules sparing the subcortical U fibers
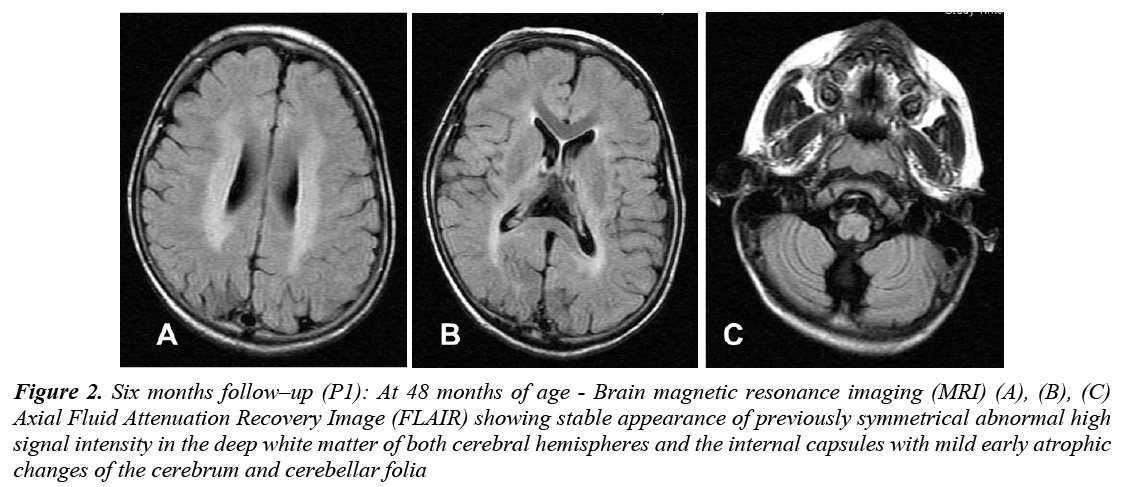
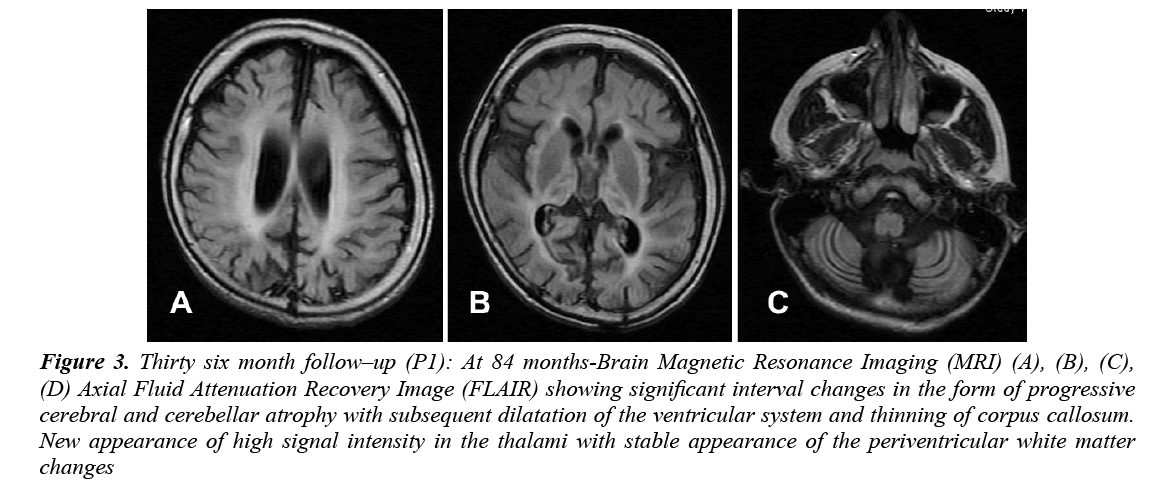
CBC, liver and renal function, glucose, thyroid functions, and basic metabolic screen were all normal. Vacuolated lymphocytes were absent. Cerebrospinal Fluid (CSF) analysis for cells, glucose, protein, lactate, amino acids and immunoglobulin was normal. Serum and CSF assay for measles antibodies were negative. Standard karyotyping was normal. Lysosomal enzyme activities in skin fibroblasts for arylsulfatase A, β-galactocerebrosidase, total hexosaminidase and hexosaminidase A were normal. EEG showed slowing of background activity with focal and generalized epileptiform discharges. VEP was normal in amplitude but delayed in latency. ERG and BAEP were all normal. Brain MRI showed static appearance of white matter changes with early cerebellar atrophy (Figure 2). Skin biopsy for electron microscope showed lipofuscine inclusion bodies. Muscle biopsy showed nonspecific changes. She received symptomatic treatment and multiple antiepileptic drugs, but she remained with intractable seizures (GTC and myoclonic). At 7 years of age, the course of the disease was marked by a complete loss of social communication. She became bedridden, blind, emaciated and needed a gastrostomy tube insertion for feeding. Brain MRI showed generalized cerebral and cerebellar atrophy with thalamic involvement (Figure 3). An opthalmological examination revealed bilateral optic atrophy, attenuation of the vessels and retinal degeneration, and ERG was moderately reduced. She remained in a vegetative state for 5 years and died at 12 years of age secondary to aspiration pneumonia.
Figure 2: Six months follow–up (P1): At 48 months of age - Brain magnetic resonance imaging (MRI) (A), (B), (C) Axial Fluid Attenuation Recovery Image (FLAIR) showing stable appearance of previously symmetrical abnormal high signal intensity in the deep white matter of both cerebral hemispheres and the internal capsules with mild early atrophic changes of the cerebrum and cerebellar folia
Figure 3: Thirty six month follow–up (P1): At 84 months-Brain Magnetic Resonance Imaging (MRI) (A), (B), (C), (D) Axial Fluid Attenuation Recovery Image (FLAIR) showing significant interval changes in the form of progressive cerebral and cerebellar atrophy with subsequent dilatation of the ventricular system and thinning of corpus callosum. New appearance of high signal intensity in the thalami with stable appearance of the periventricular white matter changes
Case Report 2
A 4 year old boy (twin I, non-identical), the younger brother of case 1 (diagnosed with vLINCL based on clinicopathological findings) presented to our clinic with psychomotor regression and unsteadiness of gait, which began at the age of 3 years and 6 months. He was a product of a full-term In Vitro Fertilization (IVF) after 6 years of infertility. Unfortunately, Preimplantation Genetic Diagnosis (PGD) was not done for this pregnancy as parents were hoping to get a normal child and did not wait for the final molecular diagnosis of the older girl. He was delivered by elective cesarean section on account of breech presentation. Natal and postnatal periods were uneventful. Although the patient`s gross and fine motor milestones were achieved within the normal ranges, his cognitive and language developments were noted to be slightly impaired. He began to lose motor milestones at 3 years and 6 months after an episode of febrile illness, with the appearance of irritability, sleeplessness, clumsiness, unsteadiness of gait and occasional drop attacks. At presentation, he was confined to a wheelchair, was irritable, normocephalic, no dysmorphism, was able to fixate and follow objects with his eyes with impairment in content and quality of speech. He had psychomotor deterioration with loss of ability to walk independently, truncal ataxia, spastic quadriparesis with difficulty in swallowing and significant drooling. Fundus examination showed pallor of the optic disc with abnormal retinal vessels and pigmentation. Systemic examination was normal, and there was no organomegaly. Basic blood tests were normal. Full metabolic screening of the serum and the urine was unrevealing. Vacuolated lymphocytes were absent. EEG showed diffuse slow background activity suggestive of encephalopathy with generalized epileptiform discharges. ERG was mildly reduced. VEP was normal in amplitude but delayed in latency. BAEP was normal. Brain MRI showed deep white matter changes with mild cerebellar atrophy. Skin biopsy for electron microscopy showed lipofuscine inclusion bodies. Muscle biopsy showed non-specific changes. He had the slowest regression in developmental milestones in comparison to his sisters. Regression mainly affected motor milestones and speech followed by progressive cognitive impairment with the appearance of multiple myoclonus, poor vision and dysphagia, with frequent chocking. Seizures developed at 5 years of age, mainly GTC and myoclonic in form. He received symptomatic treatment and multiple antiepileptic drugs. At 8 years of age, the course of the disease was marked by a complete loss of social communication; he became bedridden, blind, emaciated and needed gastrostomy tube insertion for feeding. Brain MRI showed generalized cerebral and cerebellar atrophy with thalamic involvement. An opthalmological examination revealed bilateral optic atrophy, attenuation of the vessels and retinal degeneration and ERG was moderately reduced. He remained in vegetative state for 5 years, and died at 13 years of age.
Case Report 3
A 4 years and 6 months old girl (twin II, non-identical), the youngest sister of case 1 presented to our clinic with psychomotor regression and unsteadiness of gait, which began at the age of 4 years. Her gross and fine motor milestones were achieved within the normal range and her cognitive and language developments were much better than other siblings. She began to lose motor milestones at 4 years after an episode of febrile illness post vaccination, with the appearance of irritability, sleeplessness, clumsiness, unsteadiness of gait and occasional drop attacks. At presentation she was confined to a wheelchair, was irritable, normocephalic, no dysmorphism, able to fixate and follow objects with her eyes with impairment in content and quality of speech. She had psychomotor deterioration with loss of the ability to walk independently, had truncal ataxia, spastic quadriparesis with difficulty in swallowing and significant drooling. Fundus examination showed pallor of the optic disc with abnormal retinal vessels and pigmentation. Systemic exam was normal and there was no organomegaly. Basic metabolic screening was negative. EEG showed diffuse slow background activity suggestive of encephalopathy with multifocal epileptiform discharges. ERG was mildly reduced. VEP was normal in amplitude but delayed in latency. BAEP was normal. Brain MRI showed similar findings to her brother’s. Seizures developed at 6 years of age, mainly GTC and myoclonic in form. She received symptomatic treatment and multiple antiepileptic drugs. She had the fastest regression in developmental milestones afterwards in comparison to her siblings. Regression was mainly affecting motor milestones and speech followed by a progressive cognitive impairment with the appearance of multiple myoclonus, poor vision, and dysphagia with frequent chocking. At 7 years of age, the course of the disease was marked by a complete loss of social communication. She became bedridden, blind, emaciated and needed a gastrostomy tube insertion for feeding. Brain MRI showed generalized cerebral and cerebellar atrophy with thalamic involvement. An opthalmological examination revealed bilateral optic atrophy, with macular degeneration and ERG was moderately reduced. She remained in a vegetative state for 3 years and died at 10 years of age. Molecular analysis by homozygosity mapping followed by candidate gene direct sequencing finally confirmed the clinical diagnosis of vLINCL as it revealed a nonsense homozygous mutation of CLN7 (MFSD8) (c.103 C>T, p.Arg 35X) at exon 3 in the 3 affected siblings and confirmed parental carrier state. The detailed clinical and laboratory findings are presented in Tables 1 and 2, respectively.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age at onset | 3 years | 3 years and 6 months | 4 years |
| Gender | Female | Male | Female |
| Consanguinity | + | + | + |
| Family history | Sibling | Sibling | Sibling |
| Clinical Features | |||
| Motor deterioration | + | + | + |
| Ataxia | + | + | + |
| Epilepsy | + | + | + |
| Mental deterioration | + | + | + |
| Truncal rigidity | + | + | + |
| Limb spasticity | + | + | + |
| Tremors | + | + | + |
| Visual failure | + | + | + |
| Speech impairment | + | + | + |
| Irritability/Sleep disturbance | + | + | + |
| AED | Valproic acid, Clonazepam, lamotrigine and Topiramate | Clonazepam, Topiramate and Levetiracetam | Valproic acid, Topiramate, Clonazepam and levetiracetam |
NCL: Neuronal Ceroid Lipofuscinosis; AED: Antiepileptic Drugs
Table 1: Clinical characteristics of patients with variant late infantile NCL
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Vacuolated lymphocyte | - | - | - |
| Skin biopsy | Lipofuscine pigment | Lipofuscine pigment | Lipofuscine pigment |
| Muscle biopsy | Non-specific | Non-specific | Non-specific |
| EEG | Background slowing, focal and generalized epileptiform discharges | Background slowing, generalized epileptiform discharges | Background slowing, multifocal epileptiform discharges |
| Ophthalmological evaluation | Normal retina*, optic atrophy | Retinal pigmentation, optic atrophy | Retinal pigmentation, optic atrophy |
| ERG | Normal* | Abnormal | Abnormal |
| VEP | Abnormal | Abnormal | Abnormal |
| Brain CT | Mild ventricular dilation | ND | ND |
| Brain MRI | |||
| Deep WM involvement | + | + | + |
| Thalamic involvement | + | - | - |
| Cerebral atrophy | + | + | + |
| Cerebellar atrophy | + | + | + |
| Molecular diagnosis | Homozygous mutation of CLN7 (MFSD8) | Homozygous mutation of CLN7 (MFSD8) | Homozygous mutation of CLN7 (MFSD8) |
| Outcome | Death at 12 years | Death at 13 years | Death at 10 years |
NCL: Neuronal Ceroid Lipofuscinosis; AED: Antiepileptic Drugs; EEG; Electroencephalogram; ERG: Electroencephalogram; VEP: Visual Evoked Potential; CT: Computed Tomography; ND: Not Done; MRI: Magnetic Resonance Imaging; WM: White Matter
*Detected only initially, abnormal afterwards
Table 2: Laboratory and radiological characteristics of patients with variant late infantile NCL
Discussion
A rapid clinical diagnosis of NCLs is of great importance for initiation of proper genetic counseling especially in families with consanguineous marriages. Electron microscopy (EM) has been used for diagnosing NCL and it shows unique pathological findings of ultrastructural lipofuscinic pigments in lymphocytes, cultured skin fibroblast and conjunctival or rectal biopsy. Molecular diagnosis led to CLN genotyping which may be related to specific clinical subtypes.
The patients in the present study were affected by a clear neurodegenerative disease, likely to have autosomal recessive inheritance in the presence of family consanguinity. It is characterized by an onset at 3-4 and 1/2 years of age, ataxia, followed by gradual regression of motor milestones as the first symptom, intractable seizures with progressive cognitive and speech impairment, and visual deterioration (Table 1). EM analysis of the skin biopsy showed lipofuscinic pigments (undetermined) which the major clue for the diagnosis of vLINCL was. Muscle biopsy was non-specific. In vLINCL, the ultrastructural findings of the skin have a variable interand intra-individual pattern, including the fingerprint (the most common), rectilinear, curvilinear (quite condensed, typical to CLN7 disease) and GROD pattern [10-12]. Such patterns are non-specific for precise genetic diagnosis, as other forms of vLINCL also cannot be distinguished by their ultrastructural pattern [14]. Muscle biopsy was not helpful as the typical ultrastructural features are dependent on the disease evolution and therefore may be missed when biopsy is taken early in the course of the disease, so that a repeat biopsy can be done in highly suspected cases.
The radiological findings were supportive of NCL as it showed early white matter changes, followed by progressive cerebral and cerebellar atrophy with thalamic involvement, which are well known to be associated with NCL [15-17]. The presence of thalamic and basal ganglia changes support the diagnosis of vLINCL in comparison to LINCL.
Disease course was severe with more than one seizure type occurring, including complex partial seizures, secondary generalized tonic-clonic seizures and irregular myoclonic jerks; with poor control of the later more apparent in case 1. Poor vision was noted late and attributed initially to optic atrophy, but later on blindness was caused by progressive retinal degeneration. All patients were in a vegetative state by 7-8 years of age and eventually die prematurely between 10-12 years of age. Molecular diagnosis revealed a nonsense homozygous mutation of MFSD8 at exon 3, identical to what was previously reported in Turkey, Italy and Cook Islands populations [7,10,11]. Whole – exome sequencing is another cost-effective methodological approach for establishing a rapid molecular diagnosis of Mendelian recessive diseases like NCL, particularly when mutations in distinct genes are related to overlapping phenotypes, in comparison to wide-genome autozygosity mapping which is usually time-consuming and expensive [18-20]. Mutations in MFSD8 gene were initially reported in 2007 as an important cause of vLINCL in patients of Turkish origin [12]. Since then other reports extended the list to 38 pathogenic mutations and suggested a relatively uniform severe clinical course, except for one patient with juvenile onset and protracted disease course due to the p.AIa157Pro mutation [10,11,21,22]. The Phenotypes of almost all affected individuals are very similar regardless of the mutation type and its predicted effect on the MFSD8 protein function.
Our series showed a similar phenotype to the reported Turkish variant where the mean age of disease onset ranged from 2-7 years, with seizures or motor impairment as the most common presenting symptoms, while others like vision loss developed later on, and most patients became non-ambulatory within 2 years after onset [10,23]. The features distinguishing the Turkish variant from CLN2, CLN3 included a more severe course of seizures, the presence of condensed fingerprint profiles on EM examination of lymphocytes, and lack of vacuolated lymphocytes. Mole et al. stated that the clinical phenotype of CLN7 is considered to be equivalent to the Turkish patients with CLN8 [5]. Our series showed similar features to the Egyptian family (5 members) reported by Stogmann et al., but differed in that our patients had visual impairment while no visual impairment was reported by Stogmann et al. [22], Aldahmesh et al. [20] reported Saudi individuals with vLINCL that differ from our series in that the presenting symptom was poor vision, late onset of vegetative state at 11 years, and MRI showing only atrophic changes mainly in occipital lobes, although he commented that the phenotype was similar to that in other patients with this form of CLN [21]. Our series is different from that reported by Mandel et al. [8] in that their patients had disease onset at 5-7 years, mental and motor regression at 8-10 years, and death between 14 and 23 years. Based on Kousi et al. [10] report, patients with later onset CLN should still be considered to have mutation in MFSD8 gene.
The clinical data from our patients as well as from others in the previous reported studies showed that the phenotype associated with MFSD8 gene mutations is fairly consistent, although our case series is unique in that all the affected patients are a product of intracytoplasmic in vitro fertilization, severity of the disease varies within the same family, visual loss was not the major symptom initially, early encephalopathic changes (slow background) on EEG were seen, and early white matter changes were noted on MRI. Parents received genetic counseling with advice on future Pre-Implantation Genetic Diagnosis (PGD). Current disease management is primarily targeted at controlling the symptoms rather than "curing" the disease: multiple antiepileptic drugs to control the intractable epilepsy, antispastic agents such as baclofen and benzodiazepine to control the spasticity, new antipsychotic medication, e.g. risperidone to control irritability, melatonin to control the sleep disturbance and extensive physical and occupational therapy rehabilitation. Different modalities of treatment such as anti-inflammatories, natural treatments (e.g. Antioxidants, selenium, Vit E, curcumin), enzyme replacement therapy, lysosomal modulators, stem cell therapy and gene therapy are under trial to delay or halt disease progression [24].
Conclusion
Our results expand the available information regarding variant late infantile NCL in Arab ethnicity which can be used for future genotype-phenotype correlations. The author highlights the importance of early recognition of this phenotype, and rapid confirmation by molecular diagnosis which will allow for early and proper genetic counseling including pre-implantation genetic diagnosis (PGD) and future family planning especially in communities with high rate of consanguineous marriage.
Acknowledgement
The author thanks the family for their gracious cooperation and Prof. Fowzan S. Alkuraya for his great help in confirming the diagnosis by mutational molecular studies. This work was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University.
References
- Haltia M. The neuronal ceroid-lipofuscinoses. J Neuropathol Exp Neurol 2003; 62: 1–13.
- Haltia M, Goebel HH. The neuronal ceroid-lipofuscinoses: A historical introduction. Biochim Biophys Acta 2013; 1832: 1795–1800.
- Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta 2009; 1793: 697–709.
- Anderson GW, Goebel HH, Simonati A. Human pathology in NCL. Biochim Biophys Acta 2013; 1832: 1807–1826.
- Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics 2005; 6: 107–126.
- Williams RE, Mole SE. New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology 2012; 79: 183–191.
- Kousi M, Lehesjoki AE, Mole SE. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum Mutat 2012; 33: 42–63.
- Mandel H, Cohen Katsanelson K, Khayat M, et al. Clinico-pathological manifestations of variant late infantile neuronal ceroid lipofuscinosis (vLINCL) caused by a novel mutation in MFSD8 gene. Eur J Med Genet 2014; 57: 607-612.
- Patino LC, Battu R, Ortega-Recalde O, et al. Exome sequencing is an efficient tool for variant late-infantile neuronal ceroid lipofuscinosis molecular diagnosis. PLoS ONE 2014; 9: e109576.
- Kousi M, Siintola E, Dvorakova L, et al. Mutations in CLN7/MFSD8 are a common cause of variant late-infantile neuronal ceroid lipofuscinosis. Brain 2009; 132: 810–819.
- Aiello C, Terracciano A, Simonati A, et al. Mutations in MFSD8/CLN7 are a frequent cause of variant-late infantile neuronal ceroid lipofuscinosis. Hum Mutat 2009; 30: E530-E540.
- Siintola E, Topcu M, Aula N, et al. The novel neuronal ceroid lipofuscinosis gene MFSD8 encodes a putative lysosomal transporter. Am J Hum Genet 2007; 81: 136–146.
- Sharifi A, Kousi M, Sagne C, et al. Expression and lysosomal targeting of CLN7, major facilitator superfamily transporter associated with variant late-infantile neuronal ceroid lipofuscinosis. Hum Mol Genet 2010; 19: 4497–4514.
- Williams RE, Aberg I, Autti T, et al. Diagnosis of the neuronal ceriod lipofuscinoses: An update. Biochim Biophys Acta 2006; 1762: 865-872.
- Sayit E , Yorulmaz I, Bekis R, et al. Comparison of brain perfusion SPECT and MRI findings in children with neuronal ceroid-lipofuscinosis and in their families. Ann Nucl Med 2002; 16: 201-206.
- Seitz D, Grodd W, Schwab A, et al. MR imaging and localized proton MR spectroscopy in late infantile neuronal ceroid lipofuscinosis. AJNR Am J Neuroradiol 1998; 19: 1373-1377.
- Petersen B, Handwerker M, Huppertz HI. Neuroradiological findings in classical late infantile neuronal ceroid-lipofuscinosis. Pediatr Neurol 1996; 15: 344-347.
- Bamshad MJ, Ng SB, Bigham AW, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet 2011; 12: 745–755.
- Ortega-Recalde O, Vergara JI, Fonseca DJ, et al. Whole-exome sequencing enables rapid determination of xeroderma pigmentosum molecular etiology. PLoS ONE 2013; 8: e64692.
- Alazami AM, Patel N, Shamseldin HE, et al. Accelerating novel candidate gene discovery in neurogenetic disorders via whole-exome sequencing of prescreened multiplex consanguineous families. Cell Rep 2015; 10: 148-161.
- Aldahmesh MA, Al-Hassnan ZN, Aldosari M, et al. Neuronal ceroid lipofuscinosis caused by MFSD8 mutations: a common theme emerging. Neurogenetics 2009; 10: 307-311.
- Stogmann E, El Tawil S, Wagenstaller J, et al. A novel mutation in the MFSD8 gene in late infantile neuronal ceroid lipofuscinosis. Neurogenetics 2009; 10: 73-77.
- Topcu M, Tan H, Yalnizoglu D, et al. Evaluation of 36 patients from Turkey with neuronal ceroid lipofuscinosis: clinical, neurophysiological, neuroradiological and histopathologic studies.Turk. J. Pediat 2004; 46: 1-10.
- Geraets RD, Koh SY, Hastings ML, et al. Moving towards effective therapeutic strategies for neuronal ceroid lipofuscinosis. Orphanet J Rare Dis 2016; 11: 40.