Research Article - Biomedical Research (2022) Volume 33, Issue 2
Method development and validation of Ipratropium bromide by HPLC.
Landage SS*, Shembade SH, Tamboli AM
Department of Pharmaceutical Chemistry, Sahyadri College of Pharmacy, Methvade, Maharashtra, India
- Corresponding Author:
- Landage SS
Department of Pharmaceutical Chemistry
Sahyadri College of Pharmacy
Methvade
Maharashtra
India
Accepted date: February 25, 2021
Abstract
The present work was focused on the development and validation of High-Performance Liquid Chromatography (HPLC) method which is simple, rapid, precise, accurate, sensitive, and economical for the quantitation of Ipratropium bromide in bulk and capsule dosage form has been validated. The chromatographic separation was attained on Agilent zorbax bonus-RP column with dimensions (250 × 4.6 mm, 5μ) particle size employing 0.1% Trifluoroacetic acid and Acetonitrile (ACN) in the ratio of 70:30% v/v as mobile phase, which was pumped at a rate of 1.0 ml/min and detected at a wavelength of 210 nm. The linearity of the method was demonstrated in the concentration range of 24-56 μg/ml for Ipratropium bromide with a correlation coefficient (r²) of 0.999. Percentage drug recovery was found to be 99.03%-100.08%, and percentage relative standard deviation was <2%. Limit of detection and limit of quantification values were found to be 1.15 μg/ml and 3.49 μg/ml, respectively, and assay of marketed capsule formulation was found to be 99.44%. The developed HPLC method was found to be simple, specific, sensitive, rapid, linear, accurate, precise, and economical and could be used for regular quality control of Ipratropium bromide in bulk and capsule formulation.
Keywords
Ipratropium bromide, High-performance liquid chromatography, Validation, ICH guidelines.
Introduction
Ipratropium bromide chemically known as (1R,3R,5S,8R)- 3-(3-hydroxy-2-phenylpropanoyl) oxy)-8-methyl-8-(propan-2- yl)-8-azabicyclo (3.2.1) octan-8-ium bromide is a muscarinic antagonist structurally related to atropine but often considered safer and more effective for inhalation use. It is used for various bronchial disorders, in rhinitis, and as an antiarrhythmic. It blocks muscarinic cholinergic receptors, without specificity for subtypes, resulting in a decrease in the formation of cyclic Guanosine Monophosphate (cGMP). It is freely soluble in water and methanol, sparingly soluble in ethanol, and insoluble in lipophilic solvents such as ether, chloroform and fluorocarbons [1- 7]. The combination preparation Ipratropium bromide/ salbutamol is a formulation containing Ipratropium bromide and salbutamol sulphate used in the management of Chronic Obstructive Pulmonary Disease (COPD) and asthma. An extensive literature survey revealed few RP-HPLC methods for routine quality control analysis, related substances and impurity determinations in dosage forms containing Ipratropium bromide and salbutamol sulphate. An LC-MS/MS7 method was also reported for the simultaneous determination of albuterol sulphate and Ipratropium bromide in rat plasma. An attempt has been made to develop a new RP-HPLC method for simultaneous determination of Ipratropium bromide and salbutamol sulphate in inhalations which could also provide the stability related information [8-12].
Materials and Methods
Ipratropium bromide was procured as a gift sample from Vamsi Labs Ltd. Used in the study. The pharmaceutical dosage form used in the study was Ipravent rotacaps labeled to contain 40 mcg. Ipravent rotacaps was purchased from the local pharmacy, sangola. All other chemicals and reagents used were of analytical grade.
Chromatographic condition
Chromatographic separation was performed using an Agilent zorbax bonus-RP column dimension (250 × 4.6 mm, 5μ) was used for the separation. The elution was carried out gradient at flow rate 1.0 ml/min using mobile phase consisted of 0.1% Trifluoroacetic acid: ACN (70:30 v/v).
Preparation of standard solution
Standard Stock Solution-I (SSS-I)
• Initially Prepare a Standard Stock Solution (SSS-I) of by adding 5 mg of Ipratropium bromide in 10 ml volumetric flask and add 5 ml diluents, mix for 2 minutes and make the volume to 10 ml with diluents. (Conc. of Ipratropium bromide=500 μg/ml).
• Then add 0.8 ml of SSS-I in 10 ml volumetric flask and add 5 ml diluents and vortex and make up the volume with diluents. (Conc. of Ipratropium bssromide=40 μg/ml).
Selection of wavelength
The sample was scanned from 200-400 nm with PDA detector. The Wavelength selected for analysis chosen was 210 nm on basis of appropriate intensity of Ipratropium bromide (Table 1) (Figures 1 and 2).
| Column temperature | 30º C |
| Flow rate | 1.0 ml/min |
| Mobile phase | 0.1% Trifluoroacetic acid : Acetonitrile (70:30 v/v) |
| Runtime | 10 minutes |
| Injection volume | 10 µl |
| Wavelength | 210 nm |
| Diluent | 0.1% TFA:ACN |
| Column | Agilent zorbax bonus-RP |
| RT of Ipratropium bromide | 2.50 Minutes |
Table 1: Chromatographic condition.
Sample preparation for assay
Capsule Sample Solution (CSS)
• 20 Capsule contents were weighed and average weight was calculated. And the content was mixed in mortar and pestle.
• Powder Weight equivalent to 400 μg Ipratropium Bromide was weighed into 10 ml volumetric flask and add 5 ml diluent, sonicate for 10 minutes and make the volume to 10 ml with diluent. (Conc. of Ipratropium Bromide=40 μg/ml).
Method Validation
The methods were validated as per the ICH guidelines on analytical process validation. The linearity, accuracy, precision, and specificity of the method were validated as per ICH guidelines [13-15].
Linearity
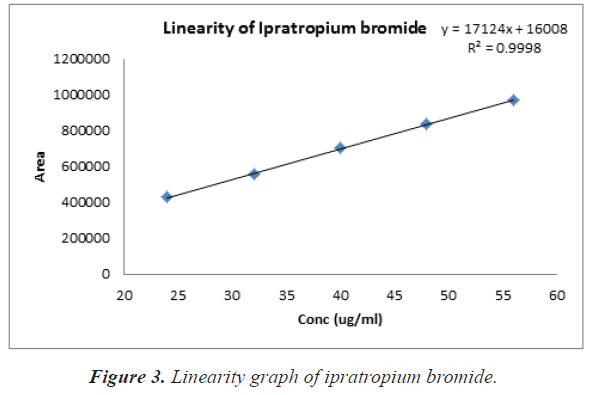
Stock solution of Ipratropium bromide at a concentration of 60%-140% was prepared in the mobiles phase. From the stock several dilutions between 24-56 μg/ml were prepared and injected into the column at a rate of 1 ml/min at injection volume of 10 μL. The calibration curve was plotted using area of retention time versus concentrations μg/ml. linear regression analysis was used to assess the linearity using least square regression method.
Precision
The assay precision was carried out by interday and intraday study and the process was evaluated for solutions (24,32,40,48,56 μg/ml) and were analyzed at different time points. The RSD (Relative Standard Deviation) was calculated for the methods.
Accuracy
Samples were prepared of 80%, 100% and 120% concentration by spiking the same amount of concentration given in Tables for Ipratropium Bromide. Samples were injected in duplicate to calculate % RSD and % recovery was also calculated.
System suitability
A single sample was prepared as described and 5 injections were made from same sample and checked for system suitability. System suitability parameters are as below
1. Retention time,
2. Theoretical plates,
3. Asymmetry (Tailing factor),
4. Resolution.
Limit of detection and limit of quantification
The Limit of Detection (LOD) and Limit of Quantification (LOQ) were determined according to ICH guidelines by the below mentioned formulae (Table 2).
| Sr. No. | % Level | Volume of Ipratropium bromide stock solution to be taken (ml) | Concentration of Ipratropium bromide (µg/ml) | Diluted to volume (ml) |
|---|---|---|---|---|
| 1. | 60 | 0.6 | 24 | 10 |
| 2. | 80 | 0.8 | 32 | 10 |
| 3. | 100 | 1.0 | 40 | 10 |
| 4. | 120 | 1.2 | 48 | 10 |
| 5. | 140 | 1.4 | 56 | 10 |
Table 2: Linearity dilutions.
LOD=3.3*SE/A
LOQ=10*SE/A
Where,
SE=Standard Error of Y intercept; A=Slope of the calibration curve.
Results and Discussion
The percentage assay of Ipratropium bromide was found to be 99.44% as shown in Table 3. Linearity was studied by plotting a graph of area vs. concentration. The calibration curve for the method obtained was linear over the concentration of Ipratropium bromide 24-56 μg/ml. The correlation coefficient obtained was 0.999, thus indicating excellent correlation between peak areas. Calibration data is presented in Table 4 and calibration curve shown in Figure 3 it proves the linearity over the concentration range 24 to 56 μg/ml. The precision study of same dilutions (40 μg/ml) of Ipratropium bromide solutions in 0.1% Trifluoroacetic acid: Acetonitrile (70:30 v/v) showed % RSD less than 2% as shown in Table 5. A good accuracy was verified with a mean recovery which was not less than 99.03% and not more than 100.08% and the range of % RSD is not less than 0.09 and not more than 0.22 for study of the different dilutions of Ipratropium bromide in 0.1% Trifluoroacetic acid: Acetonitrile (70:30 v/v) solutions as shown in Table 6. The parameter of system suitability is Retention time, Therotical plates, Assymmetry (Tailing factor) and Resolution as shown in Table 7. The lowest amount of analyte in a sample that can be detected but not necessarily quantitated LOD (Limit of Detection) and the lowest amount of analyte in a sample that can be determined with accepTable precision and accuracy under the stated experimental conditions or quantitation limit LOQ (Limit of Quantitation) was determined. The LOD and LOQ were found to be 1.15 μg/ml and 3.49 μg/ml as shown in Table 8. The significantly low value of LOD and LOQ proved the sensitivity of the process.
| Ipratropium bromide | ||
|---|---|---|
| Sample | Working standard | Drug product |
| Area | 704752 | 700786 |
| Assay | - | 99.44 |
Table 3: Assay data of Ipratropium bromide.
| % Level | Concentration (ug/ml) | Area |
|---|---|---|
| 60 | 24 | 429123 |
| 80 | 32 | 559039 |
| 100 | 40 | 704242 |
| 120 | 48 | 837803 |
| 140 | 56 | 974718 |
Table 4: Linearity data of Ipratropium bromide.
| Ipratropium bromide | ||
|---|---|---|
| Concentration of sample (µg/ml) | Sample ID | Area |
| 40 | Rep 1 | 704242 |
| 40 | Rep 2 | 706398 |
| 40 | Rep 3 | 708856 |
| 40 | Rep 4 | 702490 |
| 40 | Rep 5 | 701774 |
| Average | 704752 | |
| STDEV | 2906.775 | |
| RSD | 0.41 | |
Table 5: Precision data of Ipratropium bromide.
| Sample ID | Reps | Spiked conc. (µg/ml) |
Area | Amt. Recovered (µg/ml) |
% Recovery | Average | STDEV | RSD |
|---|---|---|---|---|---|---|---|---|
| 80% | Rep 1 | 31.99 | 559039 | 31.7233 | 99.16 | 99.03 | 0.1758 | 0.18 |
| Rep 2 | 31.99 | 557637 | 31.6437 | 98.91 | ||||
| 100% | Rep 1 | 39.99 | 704242 | 39.9630 | 99.93 | 100.08 | 0.2163 | 0.22 |
| Rep 2 | 39.99 | 706398 | 40.0854 | 100.23 | ||||
| 120% | Rep 1 | 47.99 | 837803 | 47.5421 | 99.07 | 99.13 | 0.0938 | 0.09 |
| Rep 2 | 47.99 | 838925 | 47.6058 | 99.20 |
Table 6: Accuracy data of Ipratropium bromide by HPLC method.
| Parameter | Ipratropium bromide |
|---|---|
| Retention time | 2.50 minute |
| Therotical plates | 10005 |
| Asymmetry (Tailing factor) | 1.07 |
| Resolution | 0.00 |
Table 7: System suitability parameters.
| Drug | LOD (µg/ml) | LOQ (µg/ml) |
|---|---|---|
| Ipratropium bromide | 1.15 (µg/ml) | 3.49 (µg/ml) |
Table 8: LOD and LOQ of Ipratropium bromide.
Conclusion
The developed HPLC method is able to determine Ipratropium bromide in raw materials and in pharmaceutical dosage forms; this can be attributed to the good separation and resolution of the chromatographic peaks with optimum retention time of 2.50 minutes. The results were in good conformity with the affirmed statistical and pharmacopeial contents. Hence, this method can be considered to be precise, reliable, rapid, simple, sensitive and economical nature.
Acknowledgement
The authors extend a deep sense of gratitude to management and principal of Sahyadri College of Pharmacy, methvade, sangola for providing all necessary support and facility to complete this research work.
References
- “Indian Pharmacopoeia” Government of India, Ministry of Health and Family Welfare. Published By the Indian Pharmacopoeia Commission Ghaziabad 2006; 968-970.
- Abdine HH, Belal F, Al-Badr AA. Ipratropium bromide: Physical properties. Profiles of drug substances, excipients, and related methodology 2003; 30: 59-83.
- Anjali P. Kokane, Varsha S. Tegeli, Bhagyashri S. UV-Spectrophotometric method development and validation for estimation of Ipratropium bromide in API and pharmaceutical dosage form. Int J Curr Pharm Res 2020; 12: 69-73.
[Crossref]
- Atif M, Khalid SH, Onn Kit GL, Sulaiman SAS, Asif M, Chandersekaran A. Development and validation of RP-HPLC-UV method for the determination of glipizide in human plasma. J Young Pharm 2013; 5: 26-29.
[Crossref] [Google Scholar] [PubMed]
- Beckett AH, Stenlake JB. Practical pharmaceutical chemistry fourth edition-Part two. CBS Publishers and Distributors 1997; 293-296.
- Chatwal GR. Analytical chemistry, 1st Ed. Himalaya publication house 2008; 1-3.
- Sowjanya G, Gowri Sankar D, Seshagiri Rao JV. Development and validation of a new RP-HPLC method for the simultaneous determination of albuterol sulphate and Ipratropium bromide in nasal inhalation. Int Res J Pharm 2018; 9: 63-70.
- International conference on harmonization of technical requirements for the registration of pharmaceutical for human use: Validation of analytical procedures, Text and Methodology, Q2 (R1), 2005.
- Jyothi N, Venu Gopal K, Seshagiri Rao JVLN. Development and validation of a HPLC method for the simultaneous estimation of the salbutamol sulphate and Ipratropium in inhalation dosage forms. Int J Pharm Sci 2012; 2: 79-83. [Crossref]
[Google Scholar] [PubMed]
- Kasawar GB, Farooqui. Development and validation of a stability indicating RP-HPLC method for the simultaneous determination of related substances of albuterol sulphate and Ipratropium bromide in nasal solution. J Pharm Biomed Anal 2010; 52: 19-29.
[Crossref] [Google Scholar] [PubMed]
- Nagaraju P, Appaji SCHVSS. Development and validation of novel RP-HPLC method for simultaneous estimation of levosalbutamol and Ipratropium bromide in pharmaceutical dosage forms. Int J Res Pharm Chem 2014; 4: 628-635.
- Paithankar HV. HPLC method validation for pharmaceuticals: A review. Int J Uni Pharm Bio Sci 2013; 2: 229-240.
- Ravi V, Ravi PP, Umasankar B, Sai Sneha G, Swaroopa Ch, Aparna A. RP-HPLC method for simultaneous estimation of Ipratropium bromide and levosalbutamol in pharmaceutical metered dose inhalers. Int J Res Pharm Chem 2013; 3: 112-20. 5.
- Sudha T, Krishna V, Kumar VRR. Development and validation of an analytical method for glyburide and its related compounds in tablet formulation by HPLC-UV. Turk J Pharm Sci 2014; 11: 307-316.
- ICH Harmonized Tripartite Guideline. Validation of analytical procedures: Text and methodology Q2 (R1). 1994.