- Biomedical Research (2016) Volume 27, Issue 3
Metacarpophalangeal and wrist MRI bone marrow edema can reflect the disease activity of rheumatoid arthritis and correlate with SDAI, CDAI and DAS28.
Bo Chen1#, Lei Zhang1#, Weina Guo2, Jie Han2, Xiaoqing Yang3, Peichen Shen3, Wenjian Xu4*, NingLi2*
1Department of Radiology, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, China
2Department of Rheumatology, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, China
3Department of Internal Medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120,China
4Department of Radiology, Qingdao Medical College Affiliated Hospital, Qingdao University, Qingdao 266000, China
#These authors have contributed equally to this manuscript
- *Corresponding Authors:
- Ning Li
Department of Rheumatology
Shanghai East Hospital
Tongji University School of Medicine
China
Wenjian Xu
Department of Radiology
Qingdao Medical College Affiliated Hospital
Qingdao University
China
Accepted Date: February 17, 2016
Abstract
This study aims to investigate the value of Magnetic Resonance Imaging (MRI) in detecting Bone Marrow Edema (BME) in the Metacarpophalangeal (MCP) and wrist joints for Rheumatoid Arthritis (RA) and the correlation between the degree of BME and the disease activity of RA. Nonenhanced MRI scan of dominant wrist/metacarpophalangeal joints of 60 active RA patients were scored for BME using 0.2 T extremity-MRI. The correlation between the BME scores and the disease active index (DAS28, CDAI and SDAI), Rheumatoid Factor (RF) and anti-Cyclic Cirullinated Peptide antibodies (CCP) were assessed. The intra-observer agreement was moderate to excellent, with weighted kappa ranging from 0.88 to 1.0 and 0.79 to 1.0, respectively, while the interobserver agreement ranged from 0.64 to 0.79. MRI BME of MCP and wrist joints was found in 90% RA patients. SDAI (r=0.759, P <0.01), CDAI (r=0.682, P <0.01), and DAS28 (r=0.683, P <0.01) correlated well with BME scores. No significant correlations between BME scores and RF (r=0.138, P >0.05) or anti-CCP (r=0.083, P >0.05) were existed. Metacarpophalangeal and wrist joints BME existed in a higher frequency in active RA patients by using MRI examination indicated a higher sensitivity of this method and can be used as an image indice to monitor the disease activity of RA patients.
Keywords
Rheumatoid arthritis, Bone marrow, Edema, Magnetic resonance imaging, Disease active index.
Introduction
Rheumatoid Arthritis (RA) is a chronic autoimmune disease that primarily affects joints and causes pain, stiffness, swelling and limited motion. In RA the inflammatory process leads to progressive cartilage degradation with synovial hyperplasia, change in underlying bone with erosions and high levels of pro-inflammatory mediators [1,2]. A hyperplastic synovium is the major contributor to cartilage damage in rheumatoid arthritis. Bone erosion occurs rapidly (affecting 80% of patients within 1 year after diagnosis [3]) and is associated with prolonged, increased inflammation [4]. In order to optimise therapy and patient outcomes, accurate assessment of disease activity is required. The best outcome for patients (low disease activity or remission) can be obtained by adhering to an algorithm combining optimal treatment with the most appropriate assessment of outcome, and switching therapies rapidly if the desired result is not attained [5,6]. Whilst aiming at low disease activity with intensive monitoring and rapid dose adjustments is an effective therapeutic path [7,8], a state of remission offers the greatest benefits to patients [9].
There are a number of measures that are used to assess disease activity in RA, including the Disease Activity Score (DAS), the Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), and their derivatives [10,11]. The SDAI and CDAI are simplified measures that are easier to use. The SDAI requires a simple numerical summation of five traditional core set variables: swollen and tender joint counts (using the same 28 joints that are scored in the DAS28), evaluator’s and patient’s global assessments of disease activity (in cm VAS) and CRP levels (in mg/dL) [12]; the same index without addition of CRP (CDAI) is even more practical as it uses only clinical criteria [12,13] but is equally as valid.
Imaging of inflammatory activity is of increasing importance, and among available modalities, Magnetic Resonance Imaging (MRI) seems to be one of the highest impacts. MRI will be of increasing importance to visualize joint inflammation and aid in the diagnosis, treatment and follow-up of patients with RA [14]. MRI is a noninvasive tomographic imaging technique that can produce cross-sectional images in any plane, without morphologic distortion or magnification. MRI allows direct visualization and assessment of synovitis, synovial thickening, Bone Marrow Edema (BME), tenosynovitis, cartilage destruction and bone erosion [15,16].
The periarticular BME is a frequent pathology of early and active RA. MRI is the most sensitive modality to detect BME and evaluate its degree. BME may be the most specific sign in uncontrast enhancement MRI [15]. The degree of BME can reflect the activity of inflammatory diseases. In RA bone destruction is commonly preceded by BME as shown by MRI, and the role of BME as an early marker for progressive destruction and poor functional outcome has been widely addressed [17,18]. We have applied the RA-MRI Scoring system (RAMRIS) to 0.2 T MRI imaging and detected BME in early and established RA patients. We have also investigated correlations between BME degree and disease activity of RA patients, in order to assess whether MRI can become an effective radiographic modality to monitor the disease activity of RA.
Materials and Methods
Subjects
Sixty consecutive patients with RA were recruited. All patients (42 females, 18 males; age: 52.57 ± 18.38 years; disease duration: 70.48 ± 50.12 months) fulfilled the 1987 American College of Rheumatology criteria for RA [19] and were either Rheumatoid Factor (RF) or anti-Ccyclic Citrullinated Peptide (anti-CCP) antibody positive, of whom 13 had early RA (duration <2 years) and 47 had established RA (duration 3-21 years). The patients complicated with pregnancy, viral hepatitis, diabetes mellitus, active infections or other autoimmune diseases were excluded. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Tongji University. Written informed consent was obtained from all participants.
Clinical assessment
Complete clinical and laboratory evaluations were conducted for the patients. Age, sex, disease duration, the Erythrocyte Sedimentation Rate (ESR), the C-Reactive Protein (CRP), RF and anti-CCP antibody levels and current medications were recorded. Data were collected into a predesigned form. Standardized joint counts, including Tender Joints (TJCs) and Swollen Joints (SJCs), patient’s global assessment of disease activity (PGA, VAS in cm), evaluator’s global assessment of disease activity (EGA, VAS in cm) were recorded.
Disease activity
The 3-variable DAS28 (DAS28-3) was computed using the formula [20]: DAS28=[0.56 × √T28+0.28 √SW28 × +0.70 × Ln (ESR)] × 1.08+0.16. The SDAI and the CDAI were derived as follows:
SDAI=SJC28+TJC28+EGA+PGA+CRP;
CDAI=SJC28+TJC28+EGA+PGA.
Imaging
A 0.2 Tesla dedicated-extremity MRI unit (E-Scan, Esaote, Italy) equipped with a wrist coil. All the subjects lay down in the machine and the checkpoints were in the center of the coil. The patients had MRI performed of the wrists and the second through the fifth MCP joints. All MRI examinations (wrists and MCP joints) were carried out in the coronal, axial and sagittal planes using T1-weighted echo and Short-Tau Inversion Recovery (STIR) sequences with no intravenous contrast. The T1 scanning measures were:
1. Time Repetition (TR)=650 ms, Eecho Time (TE)=26 ms
2. NEX=2
3. Field of View (FOV)=150 mm × 180 mm
4. matrix=352 freq, 256 phase (100% phase and slice FOV)
5. slice thickness=2.5-4 mm, interslice gap=0 mm.
The STIR measures were:
1. TR=1750 ms, TE=25 ms, TI=75 ms
2. NEX=2
3. FOV=150 mm × 180 mm
4. matrix=256 freq, 192 phase (100% phase FOV)
5. slice thickness=3 mm, gap=0.1 mm.
MRI evaluation
23 bones were chosen in wrist and MCP regions, including
• Distal ulnar joint
• Distal radial joint
• Central
• Lunatum
• Os trigonum
• Pisiform
• Trapezium bone
• Trapezoid bone
• Capital bone
• Hamate bone
• I-V proximal metacarpale
• II-V distal metacarpale
• II-V proximal phalanx
The first intercarpal-carpometacarpal (CMC) joint and the first MCP joint were not scored (Figure 1).
MR images were evaluated for bone edema by the same observer, using a modified OMERACT (Outcome Measures in Rheumatology) MRI scoring system (RAMRIS) [21,22] with no contrast injection. In order to determine the interreader reliability for MRI scores, images were read independently by another observer in the same manner. Both assessors were blinded to the subjects’ diagnosis.
The subjective assessment of carpal bone volume was based on the 3-D T1-weighted turbo spin-echo sequence using multiplanar reconstructions, while assessment of the bone marrow was based on all sequences. Bone marrow findings suggestive of BME were defined as a focus within the trabecular bone with ill-defined margins and signal characteristics consistent with increased water content: high signal on T2-weighted and low signal on T1-weighted images. The presence and the extension of bone marrow abnormalities were scored subjectively from 0 to 3 (0=no abnormality, 1=1-33% of the volume involved, 2=34-66% and 3=67-100% of the volume involved) for each of the following bones: distal radius and ulna (distal 1cm, epiphysis included), all carpal bones and the base (distal 1cm) of the metacarpals. A total BME-score was obtained by adding the scores for individual bones, yielding a maximum score of 69.
Statistical analysis
Analysis was undertaken using SPSS version 19. In order to evaluate the interreader reliability, the weighted kappa coefficients were calculated and differences between outcomes were evaluated using the u-test. Spearman’s correlation coefficients were calculated between MRI findings and the three disease activity index (DAS28, CDAI and SDAI), as well as RF and anti-CCP.
Results
General data
All of the 60 subjects were diagnosed as active RA, and a total of 1,380 bones were examined. The bone marrow abnormalities were seen in 54 cases (90%). The scores of the bone marrow edema in the 23 bones of each patient were from 0 to 49 (10.1 ± 1.31, 95% CI: 7.48-12.72). The SDAI was from 5.1 to 66.3 (26.07 ± 1.97, 95% CI: 22.14-30.01). The CDAI was from 5 to 56 (22.87 ± 1.8, 95% CI: 19.27-26.47). The DAS28 score was from 2.2 to 6.87 (4.33 ± 0.16, 95% CI: 4.01-4.65). The value of RF was from 2 to 2440 (210.89 ± 53.78, 95% CI: 103.28-318.50) and the value of anti-CCP was from 5 to 684.6 (220.19 ± 21.49, 95% CI: 177.19-263.19). All of the data were shown in table 1.
| Item | Values | 95% CI |
|---|---|---|
| Gender (F:M) | 42:18 | - |
| Age (years) | 52.57±18.38 | - |
| Duration (months) | 70.48±50.12 | - |
| BME scores | 10.1±1.31 | 7.48-12.72 |
| SDAI | 26.07±1.97 | 22.14-30.01 |
| CDAI | 22.87±1.8 | 19.27-26.47 |
| DAS28 | 4.33±0.16 | 4.01-4.65 |
| RF (IU/mL) | 210.89±53.78 | 103.28-318.50 |
| Anti-CCP (RU/mL) | 220.19±21.49 | 177.19-263.19 |
Table 1: The general data of 60 RA patients (n=60, x̅ ± s).
Reliability measures for MRI cartilage scores
A total of 1,380 bones were examined. The mean total BMEscore was 10.1 ± 2.83 for reader 1 and 9.8 ± 2.57 for reader 2, respectively. The intra-reader repeatability for reader 1 and reader 2 were excellent for all the 23 bones, with weighted kappa of 0.88 to 1.0 and 0.79 to 1.0, respectively. The interreader Intraclass Correlation Coefficients (ICC) was 0.90 (95% CI: 0.82 - 0.99).
MRI images and BME score
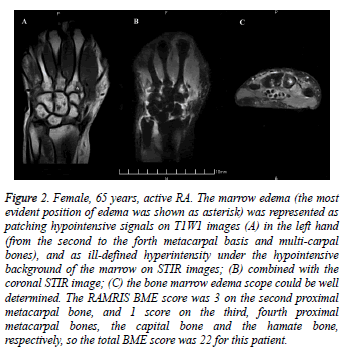
The bone marrow edema in MRI and the scores were shown in figure 2 (A-C). On MRI, the marrow edema was represented as low signal on T1W1 images, high signal on T2W1 images, and ill-defined hyper intensity under the hypo intensive background of the marrow on STIR images. The nonenhanced MRI scan could detect the marrow edema clearly. The degree of the edema could be confirmed and a score be made according to multi-plane figures. Images were scored in a blinded manner for MRI bone edema using the RAMRIS system [21].
Figure 2: Female, 65 years, active RA. The marrow edema (the most evident position of edema was shown as asterisk) was represented as patching hypointensive signals on T1W1 images (A) in the left hand (from the second to the forth metacarpal basis and multi-carpal bones), and as ill-defined hyperintensity under the hypointensive background of the marrow on STIR images; (B) combined with the coronal STIR image; (C) the bone marrow edema scope could be well determined. The RAMRIS BME score was 3 on the second proximal metacarpal bone, and 1 score on the third, fourth proximal metacarpal bones, the capital bone and the hamate bone, respectively, so the total BME score was 22 for this patient.
MRI BME scores correlated with disease activity index
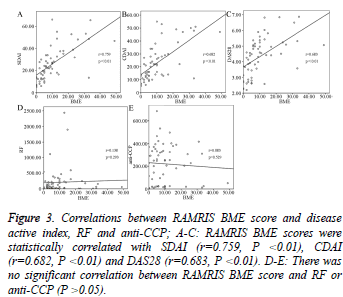
MRI BME scores (metacarpophalangeal and wrist joints) were compared with three disease activity index (SDAI, CDAI and DAS28), RF and anti-CCP. MRI BME scores were significantly positively correlated with SDAI (r=0.759, P<0.01), CDAI (r=0.682, P<0.01) and DAS28 (r=0.683, P<0.01) (Figure 3A-3C), while there was no significant correlation between BME score and RF or anti-CCP (P>0.05, Figure 3D and 3E).
Figure 3: Correlations between RAMRIS BME score and disease active index, RF and anti-CCP; A-C: RAMRIS BME scores were statistically correlated with SDAI (r=0.759, P <0.01), CDAI (r=0.682, P <0.01) and DAS28 (r=0.683, P <0.01). D-E: There was no significant correlation between RAMRIS BME score and RF or anti-CCP (P >0.05).
Discussion
Rheumatoid Arthritis (RA) leads to destruction of cartilage and bone, also affects the adjacent bone marrow, which is an additional compartment in the disease process of RA [23]. It’s important for rheumatologists to have correct information about the degree of inflammatory activity as a basis for diagnostic and therapeutic decisions. Radiography is a standard imaging technique for assessing destructive joint lesions in RA [24]. It is one of the few objective criteria used for diagnosis of this disease as part of the American College of Rheumatology (ACR) 1987 revised criteria for the classification of RA [19]. MRI is a sensitive modality that can assess both inflammatory and structural lesions, which is known to be considerably more sensitive than conventional radiography and clinical examination for the detection of RA joint pathology such as bone erosions, osteitis, or synovitis [25,26]. It has also provided new information about the early features of RA, and high scores for synovitis, tenosynovitis, bone edema and erosions may occur in up to 45% of patients within 6 months of symptom onset [25]. Some studies have shown that bone marrow edema may be predictive of progression in certain RA populations, and the bone erosion is driven by osteitis (bone edema on MRI) [27,28]. MRI is well established as an important imaging modality in the assessment of joint inflammation and damage in RA.
The rheumatoid arthritis MRI score (RAMRIS) [21] has allowed the various pathologies within the rheumatoid joint to be quantified including synovitis, bone edema and bone erosion. Recent evidence from MRI studies, however, suggested the possibility of a bone marrow involvement in RA. Thus, bone marrow alterations commonly termed as “bone marrow edema” with high signal intensity on STIR or T2- weighted fat suppressed images and low signal intensity on T1- weighted images, are present in patients with RA [21,29]. Extending these observations, another study provides direct histological evidence for cortical penetration and bone marrow changes in RA [23]. BME has recently been demonstrated to represent the strongest independent predictor of radiographic progression in hands, wrists and forefeet after 2-year disease duration in RA [30].
We assessed the practical usefulness of MRI in establishing a positive RA diagnosis by detecting the bone marrow edema. A higher positive rate of bone marrow edema (90%) could be found by this method in early and active RA patients, consistent with previous reports [31-33]. Although accurate assessment of RA disease activity is one of the most important aspects of caring for RA patients, conducting the assessments can be complex in the clinical setting [10]. The formula to calculate the DAS28 score is complicated, requiring a calculator or computer program. The simplified measures (i.e. the SDAI and CDAI) perform at least as well as the complex indices to evaluate disease activity and response to treatment, are easier to calculate, and more stringent in the definition of remission. These assessment measures are important in routine practice as they allow physicians to evaluate the success (or otherwise) of therapy by determining whether patients have achieved low disease activity or remission, and to adapt therapy as required in order to attain these goals [34]. Both the SDAI and CDAI have been validated [10], and both also have documented/validated cut-off points for categories of disease activity (high, moderate, low, and remission) which are highly correlated with the degree of joint damage [9,35,36]. The SDAI and CDAI strongly correlate with the DAS28, and like the DAS28, moderately correlate with Health Assessment Questionnaire-Disability Index (HAQ-DI) and radiographic score [9,12,35,36]. All of these indices provide an accurate assessment of disease activity in patients with high-tomoderate RA disease activity. However, when remission is addressed, the SDAI and CDAI are more stringent than the DAS28, since the latter allows for more than 10 residual swollen joints in its classification of remission [36,37]. Besides, the SDAI and CDAI are the only scores which do not require a calculator or computer.
In terms of composite measures, the SDAI was found to be more discriminating than the DAS28 (using either measures of CRP or ESR). Furthermore, of all the variables studied (including the DAS28), the SDAI most closely correlated with the physicians’ decisions to change therapy. This was further confirmed in an ACR initiative spearheaded by Felson, which showed that among numerous tools and their variations, the decisions to change therapy were best associated with changes in SDAI and CDAI [38]. In this study, statistically significant correlations were observed between MRI BME scores and SDAI, CDAI, and DAS28, suggesting these index can be used good predictive factors for RA activity. The relatively stronger correlation observed between SDAI (includes CRP) and MRI scores could be due to the objectivity of these measures.
Interestingly, the lack of correlation between RAMRIS bone edema scores and clinical items, including RF and anti-CCP, could be due to the higher sensitivity of MRI versus RF and anti-CCP in detecting bone inflammation. Besides, although IgM-RF was associated with disease activity, anti-CCP and IgA-RF, IgG-RF was not associated with disease activity [39]. If the images were done in only 1 (coronal) plane, there may exist a false-positive [31]. We applied three planes (coronal, axial and sagittal) in our study, in order to get a more accurate result. Overall, the correlations between RAMRIS bone edema scores and evaluated disease activity index indicated that MRI findings represent disease activity as measured by conventional methods, thus confirming the findings of previously reports [22,40].
Conclusion
In conclusions, the sensitivity of MRI in detecting bone marrow edema was higher and easy to perform. Bone marrow edema of RA patients was shown as inequitable hypointensity in T1W1 sequence and ill-defined hyperintensity in T2W1 and STIR sequences, especially displayed better in STIR sequence. There was a higher positive rate and sensitivity of bone marrow edema in active RA patients. There existed a significantly positive correlation between the carpometacarpal bone marrow edema degree and the disease activity. MRI bone edema score may be used as an objective index to assess the disease activity of RA and will be helpful to evaluate the patients’ condition accurately and predict the prognosis of the disease. A longitudinal follow-up of our patient cohort is planned to define the treatment effect of bone marrow edema and its relationship to the other features of rheumatoid pathology.
Acknowledgements
This work was supported in part by national natural science foundation (81401344), Shanghai natural science foundation (13ZR1433900).
References
- Vincenzi F, Padovan M, Targa M, Corciulo C, Merighi S. A2A Adenosine Receptors Are Differentially Modulated by Pharmacological Treatments in Rheumatoid Arthritis Patients and Their Stimulation Ameliorates Adjuvant-Induced Arthritis in Rats. PLoS One 2013; 8: e54195.
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011; 365: 2205-2219.
- van der Heijde DM. Joint erosions and patients with early rheumatoid arthritis. Br J Rheumatol 1995; 34: 74-78.
- Visser H, le Cessie S, Vos K, Breedveld FC, Hazes JM. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum 2002; 46: 357-365.
- Martin WJ, Shim M, Paulus HE, Chaudhari S, Feng J. Older Age at Rheumatoid Arthritis Onset and Comorbidities Correlate With Less Health Assessment Questionnaire-Disability Index and Clinical Disease Activity Index Response to Etanercept in the RADIUS 2 Registry. J Clin Rheumatol 2014; 20: 301-305.
- Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014; 370: 2377-2386.
- Grigor C, Capell H, Hall S, Wilkinson B, Bradley JD. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004; 364: 263-269.
- de Vries-Bouwstra J, Le Cessie S, Allaart C, Breedveld F, Huizinga T. Using predicted disease outcome to provide differentiated treatment of early rheumatoid arthritis. J Rheumatol 2006; 33: 1747-1753.
- Smolen JS, Han C, van der Heijde DM, Emery P, Bathon JM, ASPIRE Study Group. Radiographic changes in rheumatoid arthritis patients attaining different disease activity states with methotrexate monotherapy and infliximab plus methotrexate: the impacts of remission and tumour necrosis factor blockade. Ann Rheum Dis 2009; 68: 823-827.
- Martins FM, da Silva JA, Santos MJ, Vieira-Sousa E, Duarte C. DAS28, CDAI and SDAI cut-offs do not translate the same information: results from the Rheumatic Diseases Portuguese Register Reuma.pt. Rheumatology (Oxford) 2015; 54: 286-291.
- da Mota LM, Laurindo IM, de Carvalho JF, dos Santos-Neto LL. Prognostic evaluation of early rheumatoid arthritis. Swiss Med Wkly 2010; 140: w13100.
- Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003; 42: 244-257.
- Aletaha D, Smolen JS. The definition and measurement of disease modification in inflamma-tory rheumatic diseases. Rheum Dis Clin North Am 2006; 32: 9-44.
- Hammer HB, Haavardsholm EA. Advances in imaging. Curr Opin Rheumatol 2012; 24: 299-305.
- Narváez JA, Narváez J, De Lama E, De Albert M. MR imaging of early rheumatoid arthritis. Radiographics 2010; 30: 143-165.
- Olech E, Crues JV, Yocum DE, Merrill JT. Bone marrow edema is the most specific finding for rheumatoid arthritis (RA) on noncontrast magnetic resonance imaging of the hands and wrists: a comparison of patients with RA and healthy controls. J Rheumatol 2010; 37: 265-274.
- Lisbona MP, Pàmies A, Ares J, Almirall M, Navallas M. Association of bone edema with the progression of bone erosions quantified by hand magnetic resonance imaging in patients with rheumatoid arthritis in remission. J Rheumatol 2014; 41: 1623-1629.
- van Steenbergen HW, van Nies JA, Huizinga TW, Reijnierse M, van der Helm-van Mil AH. Subclinical inflammation on MRI of hand and foot of anticitrullinated peptide antibody-negative arthralgia patients at risk for rheumatoid arthritis. Arthritis Res Ther 2014; 16: R92.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF. The American Rheumatism Association 1987 revised criteria for the classifi cation of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315-324.
- Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38: 44-48.
- Østergaard M, Peterfy C, Conaghan P, McQueen F, Bird P. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol 2003; 30: 1385-1386.
- Emery P, van der Heijde D, Ostergaard M, Conaghan PG, Genovese MC. Exploratory analyses of the association of MRI with clinical, laboratory and radiographic findings in patients with rheumatoid arthritis. Ann Rheum Dis 2011; 70: 2126-2130.
- Jimenez-Boj E, Redlich K, Türk B, Hanslik-Schnabel B, Wanivenhaus A. Interaction between synovial inflammatory tissue and bone marrow in rheumatoid arthritis. J Immunol 2005; 175: 2579-2588.
- van Nies JA, van Steenbergen HW, Krabben A, Stomp W, Huizinga TW. Evaluating processes underlying the predictive value of baseline erosions for future radiological damage in early rheumatoid arthritis. Ann Rheum Dis 2015; 74: 883-889.
- Axelsen MB, Eshed I, Hørslev-Petersen K, Stengaard-Pedersen K, Hetland ML, OPERA study group. A treat-to-target strategy with methotrexate and intra-articular triamcinolone with or without adalimumab effectively reduces MRI synovitis, osteitis and tenosynovitis and halts structural damage progression in early rheumatoid arthritis: results from the OPERA randomised controlled trial. Ann Rheum Dis 2015; 74: 867-875.
- McQueen FM. Imaging in early rheumatoid arthritis. Best Pract Res Clin Rheumatol 2013; 27: 499-522.
- McQueen FM, Gao A, Ostergaard M, King A, Shalley G. High-grade MRI bone oedema is common within the surgical field in rheumatoid arthritis patients undergoing joint replacement and is associated with osteitis in subchondral bone. Ann Rheum Dis 2007; 66: 1581-1587.
- McQueen F, Clarke A, McHaffie A, Reeves Q, Williams M. Assessment of cartilage loss at the wrist in rheumatoid arthritis using a new MRI scoring system. Ann Rheum Dis 2010; 69: 1971-1975.
- McQueen FM, Benton N, Perry D, Crabbe J, Robinson E. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum 2003; 48: 1814-1827.
- Hetland ML, Ejbjerg B, Hørslev-Petersen K, Jacobsen S, Vestergaard A, CIMESTRA study group. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA). Ann Rheum Dis 2009; 68: 384-390.
- Krabben A, Stomp W, van Nies JA, Huizinga TW, van der Heijde D. MRI-detected subclinical joint inflammation is associated with radiographic progression. Ann Rheum Dis 2014; 73: 2034-2037.
- Conaghan PG, Emery P, Østergaard M, Keystone EC, Genovese MC. Assessment by MRI of inflammation and damage in rheumatoid arthritis patients with methotrexate inadequate response receiving golimumab: results of the GO-FORWARD trial. Ann Rheum Dis 2011; 70: 1968-1974.
- Boesen M, Kubassova O, Bouert R, Axelsen MB, Ostergaard M. Correlation between computer-aided dynamic gadolinium-enhanced MRI assessment of inflammation and semi-quantitative synovitis and bone marrow oedema scores of the wrist in patients with rheumatoid arthritis--a cohort study. Rheumatology 2012; 51: 134-143.
- Smolen JS, Aletaha D. Activity assessments in rheumatoid arthritis. Curr Opin Rheumatol 2008; 20: 306-313.
- Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005; 23: S100-108.
- Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum 2005; 52: 2625-2636.
- Landewé R, van der Heijde D, van der Linden S, Boers M. Twenty-eight-joint counts invalidate the DAS28 remission definition owing to the omission of the lower extremity joints: a comparison with the original DAS remission. Ann Rheum Dis 2006; 65: 637-641.
- ACR Committee to Reevaluate Improvement Criteria. A proposed revision to the ACR20: The hybrid measure of American College of Rheumatology response. Arthritis Rheum 2007; 57: 193-202.
- Gu YL, Chen YM, Lv L, Xu ML, Zhou HJ. The study of anti-cyclic citrullinated peptide antibodies and rheumatoid factors with the disease activity of rheumatoid arthritis. Chin J Rheumatol 2005; 9: 681-683.
- Hodgson RJ, O'Connor P, Moots R. MRI of rheumatoid arthritis image quantitation for the assessment of disease activity, progression and response to therapy. Rheumatology (Oxford) 2008; 47: 13-21.