Research Article - Biomedical Research (2017) Volume 28, Issue 8
Metabolomics analysis of human pancreatic cancer tissue and paired adjacent tissue samples
Dong Ouyang* and Qingyue DaiDepartment of General Surgery, Armed Police Corps Hospital in Fujian, Fuzhou, PR China
- *Corresponding Author:
- Dong Ouyang
Department of General Surgery
Armed Police Corps Hospital
PR China
Accepted date: January 02, 2017
Abstract
Background/aims: This study aimed to apply 1H spectrum Nuclear Magnetic Resonance spectroscopy (1H-NMR) to detect the metabolites in pancreatic cancer tissue and paired adjacent tissue samples.
Methods: 48 samples were taken from cancer tissues of 24 pancreatic cancer patients and paired adjacent tissues. The samples were subjected to 1H-NMR and principle component analysis to compare endogenous small-molecule metabolites.
Results: Leucine and isoleucine levels were significantly higher in pancreatic cancer tissues than in control tissues. Lactate, betaine, choline, α-glucose and α-hydroxybutyrate levels were significantly lower in pancreatitic cancer tissues than in control tissues.
Conclusions: 1H-NMR-based metabolomics is an effective method to identify small-molecule metabolites in pancreatic cancer tissues. These metabolites can be used as the metabolic markers of pancreatic cancer for early diagnosis.
Keywords
Metabolomics, 1H-NMR, Extracted liquid, Pancreatic cancer, Metabolic markers
Introduction
Pancreatic cancer is one of the most serious malignant tumors with low radical operation rate and high mortality. For pancreatic cancer patients, the median survival is only 3 to 6 months and five year survival rate is less than 5% [1-4]. In United States 48,960 people were estimated to be diagnosed with pancreatic cancer, and 40,560 people were estimated to die of pancreatic cancer in 2015 [5]. Although the incidence of pancreatic cancer is lower in China than Western countries, it increases rapidly in recent years. 34,509 men and 23,226 women died of pancreatic cancer in 2010 [6-8].
The etiology of pancreatic cancer is unknown and it is difficult for early diagnosis of pancreatic cancer. In this study, we used 1H-NMR to identify low molecular metabolites of pancreatic cancer tissues and paired adjacent tissues from patients with pancreatic cancer. Furthermore, we performed principal component analysis to explore the characteristic metabolites of pancreatic cancer which will provide a new approach for early diagnosis of pancreatic cancer.
Materials and Methods
Clinical samples
Tissue samples were collected from pancreatic cancer patients who visited Department of General Surgery, including 24 samples of pancreatic cancer tissues and paired 24 samples of tumor adjacent non-tumor pancreatic tissues. Intraoperative rapid frozen pathology confirmed tumor tissues and adjacent tumor free pancreatic tissues. All pancreatic cancer patients were diagnosed by pathological examination.
NMR and principal component analysis
Tissue extracts were prepared as described previously [9]. The samples were lyophilized or nitrogen dry. For NMR analysis, water soluble extract samples were kept at 25°C on a Bruker avance DRX 600 NMR spectrometer (Bruker, Germany), using one-dimensional pre-saturation 1D NOESY spectra (NoesyPr1d). The spectrometer was equipped with three resonance probe and Z axis pulse gradient field, the proton resonance frequency was 600.13 MHz. Spectrum data were analysed using principal component analysis.
Results
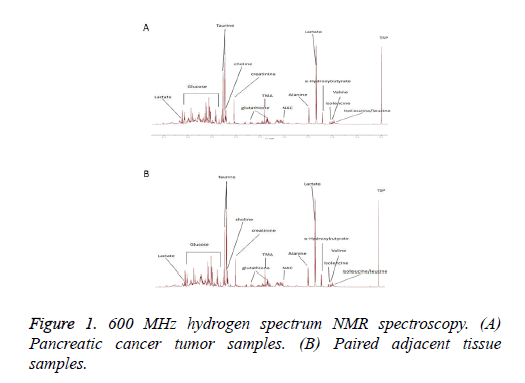
1H NMR spectroscopy analysis of endogenous metabolites of pancreatic cancer and paired adjacent tissue samples were shown in Figure 1. Table 1 listed the metabolites that showed significant changes between pancreatic cancer and paired adjacent tissue samples.
| Metabolites | Main characteristic peak chemical shift | Cancer vs. adjacent to cancer |
|---|---|---|
| Leucine | 0.942-0.961 | ↑ |
| Isoleucine | 0.950-0.982 | ↑ |
| Lactate | 1.311-1.332 | ↓ |
| Betaine | 3.684 | ↓ |
| Choline | 4.079 | ↓↓ |
| Α-glucose | 3.406-5.243 | ↓ |
| α-Hydroxybutyrate | 1.195-1.215 | ↓↓ |
| ↑, ↓ p<0.05; ↓↓ p<0.01. | ||
Table 1: List of the metabolites with significant changes between pancreatic cancer and paired adjacent tissue samples.
Discussion
Because of unnoticeable symptoms and deep location, pancreatic cancer often develops silently without symptoms at early stage. At present, the diagnosis methods of pancreatic cancer include: (1) Tumor markers: current markers lack sensitivity and specificity for early lesions. (2) Gene detection: current clinical trials have not yet found a specific gene for pancreatic cancer. (3) Imaging diagnosis: ultrasonic gastroscopy and Endoscopic Ultrasonography guided Fine Needle Aspiration biopsy (EUS guided FNA), Positron Emission Computed Tomography (PET-CT) and Multislice Spiral Computed Tomography (MSCT) and Magnetic Resonance Imaging (MRI). Imaging technologies lack the sensitivity and specificity of the tumor with a diameter smaller than 2 cm.
Hydrogen spectrum based nuclear magnetic resonance is a new approach for qualitative and quantitative analysis of metabolomics of the organism or cell. The present study was to explore the use of hydrogen spectrum nuclear magnetic resonance spectroscopy to identify low molecular weight metabolites in pancreatic cancer tissue. Using principal component analysis, we found that leucine and isoleucine levels were significantly higher while lactate, betaine, choline, alpha-glucose, alpha-hydroxybutyrate levels were significantly lower in pancreatic cancer tissues compared to adjacent non-tumor pancreatic tissues.
Leucine and isoleucine are essential amino acids. Leucine and isoleucine play an important role in the metabolism of glucose in the liver by downregulating gluconeogenesis and inhibiting the expression of rate limiting enzyme for gluconeogenesis [10]. Pancreatic carcinoma tissues had high levels of leucine and isoleucine compared with adjacent tissues which had active glucose metabolism. Furthermore, alpha-glucose level was lower in tumor tissues than the paired adjacent tissues, consistent with the results of leucine and isoleucine.
Several studies have suggested the link of betaine intake and reduced risk of nasopharyngeal carcinoma and colorectal cancer [11,12]. This is in agreement with our results that the level of betaine in pancreatic cancer tissue was significantly lower than that in the adjacent tissues.
Serum alpha hydroxy acid levels increased in diabetes. Varvel et al. found that the potential cause of the rapid increase in the serum levels of alpha hydroxy acid was the damage of insulin secretion [13]. Many studies have found a correlation between pancreatitis, pancreatic cancer and diabetes. In this study, the level of alpha hydroxy acid in pancreatic cancer tissue was significantly lower than that in the adjacent tissues.
Lactic acid is the product of anaerobic fermentation. Tumor tissue is prone to hypoxia, ischemia and blood supply deficiency, and the level of lactic acid increased significantly in many tumor tissues [14,15]. However, in the present study we found that the level of lactate in the extract of pancreatic cancer tissue did not increase. Further studies are needed to understand the underlying mechanism.
In conclusion, this study showed that endogenous small molecule metabolites of pancreatic cancer tissue and paired adjacent tissue samples were significantly different. Application of hydrogen spectrum NMR metabolic group method to explore the characteristics of low molecular metabolites in the tissue extracts of pancreatic cancer patients with pancreatic carcinoma tissues and paired adjacent tissue can provide a new approach for early diagnosis of pancreatic cancer.
References
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 1039-1049.
- Zell JA, Rhee JM, Ziogas A, Lipkin SM, Anton-Culver H. Race, socioeconomic status, treatment, and survival time among pancreatic cancer cases in California. Cancer Epidemiol Biomarkers Prev 2007; 16: 546-552.
- Lau MK, Davila JA, Shaib YH. Incidence and survival of pancreatic head and body and tail cancers: a population-based study in the United States. Pancreas 2010; 39: 458-462.
- Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971-2011: a population-based study. Lancet 2015; 385: 1206-1218.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5-29.
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France International Agency for Research on Cancer 2013.
- Chen W, Zheng R, Zhang S, Zhao P, Zeng H. Report of cancer incidence and mortality in China, 2010. Ann Transl Med 2014; 2: 61.
- Olaf B, Hector CK, Timothy MDE, Jacob B, Elaine H, John CL, Jeremy KN. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc 2007; 2: 2692-2703.
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277-300.
- Doi M, Yamaoka I, Nakayama M, Sugahara K, Yoshizawa F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am J Physiol Endocrinol Metab 2007; 292: E1683-1693.
- Zeng FF, Xu CH, Liu YT, Fan YY, Lin XL. Choline and betaine intakes are associated with reduced risk of nasopharyngeal carcinoma in adults: a case-control study. Br J Cancer 2014; 110: 808-816.
- Lu MS, Fang YJ, Pan ZZ, Zhong X, Zheng MC. Choline and betaine intake and colorectal cancer risk in Chinese population: a case-control study. PLoS One 2015; 10: e0118661.
- Varvel SA, Pottala JV, Thiselton DL, Caffrey R, Dall T. Serum α-hydroxybutyrate (α-HB) predicts elevated 1 h glucose levels and early-phase α-cell dysfunction during OGTT. BMJ Open Diabetes Res Care 2014; 2: e000038.
- Schwickert G, Walenta S, Sundfør K, Rofstad EK, Mueller-Klieser W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res 1995; 55: 4757-4759.
- Walenta S, Chau TV, Schroeder T, Lehr HA, Kunz-Schughart LA, Fuerst A, Mueller-Klieser W. Metabolic classification of human rectal adenocarcinomas: a novel guideline for clinical oncologists? J Cancer Res Clin Oncol 2003; 129: 321-326.