- Biomedical Research (2005) Volume 16, Issue 2
Megakaryocyte morphometry in chronic myeloid leukaemia and thrombocytopenic purpura
Surya Vasishta Tatapudi, Debdatta Basu*Department of Pathology, JIPMER, Pondicherry, India
- *Corresponding Author:
- Dr. Debdatta Basu
DII/18, JIPMER Campus, Pondicherry 605006, India
Phone: 0091-413-2274362
E-mail: ddbasu ( at ) satyam.net.in
Accepted date: July 8, 2005
Abstract
Morphologic and morphometric assessment of megakaryocytes was performed in 15 cases each of chronic myeloid leukemia and idiopathic thrombocytopenic purpura and compared with 15 normal cases. The average size of megakaryocytes was 174.66 μ2 in CML and 407.56μ2 in ITP, which were significantly different than that of normal. There was marked heterogeneity in size and topographic distribution and location of megakaryocytes in CML than in ITP and normal marrow. Megakaryocyte size was also different in differing grades of fibrosis in the marrow. Morphometry of megakaryocytes is a simple tool that may be useful in the diagnosis of disorders affecting megakaryocyte lineage.
Keywords
Megakaryocyte, Morphometry, Chronic Myeloid leukemia, Idiopathic Thrombocytopenic Purpura, bone marrow
Introduction
Chronic Myeloid Leukemia (CML) involves neoplastic conversion and transformation of multipotent progenitor cells. Cell lineages involved to a variable extent in CML consist not only of granulocytes, but also megakaryocytes, and at times erythroid cells and macrophages [1]. Studies have shown that size, mor-phology and number of megakaryocytes differ in different cases of CML [2-5]. Idiopathic Thrombocytopenic Purpura (ITP) is another condition associated with megakaryocyte hyperplasia. However the size and morphology of megakaryocytes in ITP differs from those in CML [4,5]. Precursor megakaryocyte size can be estimated in normal and pathological bone marrow sec-tions by morphometric techniques [2,6].
The objectives of the present study were to study the size, type and distribution of megakaryocytes and perform morphometric analysis of megakaryocytes in cases of CML and ITP and to compare them with normal marrow.
Materials and Methods
Cases were recruited from records kept in the hematology section of the department of pathology and included diagnosed cases of CML and ITP. The diagnosis was based on detailed clinical findings and hematologic and other laboratory investigations, according to standard protocols.
Bone marrow biopsy sections were retrieved form the records of pathology department. Fifteen cases each of CML and ITP were studied and fifteen normal bone marrow biopsies served as controls. Patients with normal marrow and no hematologic abnormality were selected as controls. These were the cases which were set apart in order to perform staging for lymphoma. Only those bone marrow biopsies, which were at least 1.5cm in length and had at least 4 marrow spaces, were selected for study. Each biopsy was 4-5μ in thickness and had been stained with Hematoxylin and Eosin stain, Reticulin and Masson Trichrome. Each slide was coded and the authors were not aware of the diagnosis at the time of morphometry. The cellularity, predominant cell type and megakaryocyte size and distribution were the parameters analyzed. Morphometry was done using an ocular micrometer placed in the eyepiece with the ob-jective set at a magnification of 40x. The maximum and mini-mum dimensions of at least ten megakaryocytes were meas-ured from each bone marrow specimen. Reticulin and Masson Trichrome stains were used to grade fibrosis into grades as follows- I, II, IIIa, IIIb. Statistical analysis was done to compare the size of megakaryocytes in CML, ITP and Normal by stu-dents unpaired – T test. P value<0.05 was taken as significant.
Results
Fifteen cases each of CML and ITP were studied and fifteen normal bone marrow biopsies served as controls during the course of the study. The following are the observations made.
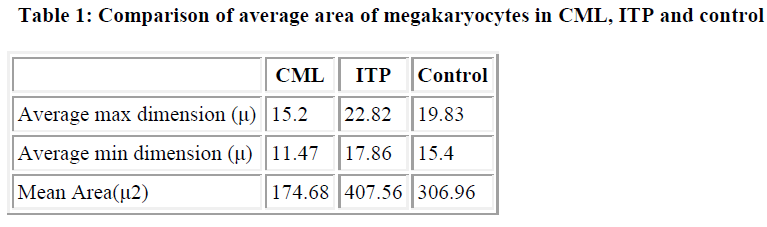
A study of bone marrow biopsy in CML revealed hypercellularity with myeloid hyperplasia and suppression of erythropoiesis. An increase in number of megakaryocytes was seen in 12 cases, while they were normal in number in two and decreased in one. Most of the megakaryocytes were small in size with a small and multilobed nucleus and granular cytoplasm. In some cases however, the megakaryocytes were large with hyperchromatic and pleomorphic nuclei. Many cases also showed a few imma-ture forms with a single round nucleus. In six cases, the megakaryocytes were scattered while in others they were clustered and in two were in sheets. Morphometric analysis showed that megakaryocytes ranged in size from 10.5μm to 20.25μm in the maximum dimension and from 8μm to 15.25μm in the minimum dimension. Average area of megakaryocyte was 174.66 μ2. Of the fifteen cases studied, six cases had low-grade fibrosis of grades I and II and five cases had grade IIIa fibrosis. Collagenisation (grade IIIb) was seen in four cases.
The bone marrow picture in cases of ITP was hypercellular with normal myelopoiesis and erythropoiesis. Megakaryopoiesis was found to be increased and the cells were large in size with smaller and unilobed nuclei. The megakaryocytes were scat-tered diffusely in all the cases. The size of the megakaryocytes ranged from 19.75μm to 25μm in the maximum dimension with a mean of 22.86μm. It ranged from 10.75μm to 22.75μm in the minimum dimension with a mean of 17.86μm. The average area of a megakaryocyte was 407.56μ2. Grade I fibrosis was noted in 14 cases while it was grade 2 in one.
In the control specimens, bone marrow ranged from normocellular to hypercellular. Hematopoiesis was essentially normal except in four cases where there was eosinophilia. Megakaryo-cytes appeared normal in morphology and distribution in all cases. Morphometric analysis showed that megakaryocytes ranged in size from 13.75μ to 26.5μ in the maximum dimension with a mean of 19.83μ. In the minimum dimension it ranged from 10.75μ to 21.75μ with a mean of 15.48μ. Average area of megakaryocyte was 306.96μ2. Grade 1 fibrosis was seen in all cases.
A comparison was made between the average area of mega-karyocyte in CML, ITP and control (Table 1). The differences was found statistically significant (P<0.001)
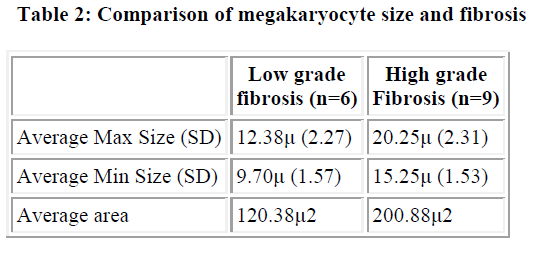
Comparison was also made between the size of megakaryocyte and the degree of fibrosis, in CML. There were 6 cases with low-grade fibrosis (grade 1 and 2) and 9 cases with high-grade fibrosis (grade 3a and 3b). The maximum dimension of mega-karyocyte in low-grade fibrosis was 12.38μ and minimum was 9.70μ while in high-grade it was 20.25μ and 15.25μ respectively. The mean area in low-grade fibrosis was 120.13μ2 while it was 200.88μ2 in high-grade (Table 2). This difference was statistically significant (p <0.001)
Discussion
Bone marrow morphology in CML is characterised by marked proliferation of megakaryocytes, which reveal distinctive features [5]. Different types of normal megakaryocytes and abnormal megakaryocytes have been recognised in all groups of chronic myeloproliferative disease [6-9]. Two histological proc-esses exert a major influence on the natural history of CML: the myeloid cell lines and the megakaryocytes [6]. Fibrosis that is often a very important component of CML originates from a disturbance in megakaryocytes and platelets [5,6]. Because megakaryopoiesis plays such a pivotal role in the disease process, a better evaluation of both the morphology and function of mega-karyocytes is warranted in CML.
Working classification of CML as proposed by Bartl et al, divides the entity into two morphologic subgroups based on the bone marrow features: 1) CML- granulocytic type, with a predominance of myeloid cells and a micromegakaryocytes which are distributed normally and 2) CML– granulocytic megakaryocytic type with a striking increase in megakaryocytes which are clustered and display morphologic heterogeneity [10]. This classification has prognostic significance. The granulocytic type is prone to blastic transformation while the megakaryocytic type develops into a fibrotic transformation. In a study of sixty patients of CML followed up for 30 months, 25% of patients who had features of CML-granulocytic type went in to blast crisis while none of the patients of CML- granulocytic megakaryocytic type developed blast crisis [11].
Morphometric analysis of sections of biopsy specimens from patients with myeloproliferative disorders can complement the individual histological diagnosis and help to distinguish the vari-ous subgroups [1,3,4,12]. Morphological characteristics of the different subtypes of CML have been derived by applying im-munohistochemical and morphometric techniques to bone marrow biopsies and to combine these results with relevant clinical parameters [3].
Morphometry has been earlier done using routine H&E as well as immunohistochemistry which highlights the megakaryocytes better [3,4,12]. In the present study morphometry was done on routine H&E stained sections. Both the authors agreed upon the identity of megakaryocytes observed during the study. Mor-phometry was done on a minimum of ten megakaryocytes in each case. The average size of megakaryocytes in CML was 174.68μm2. This is comparable to the study performed by Oh-shima et al who obtained a mean area of 263.8μm2 [4]. They had found a striking increase in size in the other types of mye-loproliferative disorders like essential thrombocythemia. In the present study we did not include any other types of CMPD due to the low number of cases.
In the present study, the size of megakaryocytes in CML differed from that of normal. CML is a disease where mega-karyocyte morphology exerts a significant influence on the natu-ral history. In our study we noticed a marked variation in the dimensions of megakaryocytes with dimensions ranging from 8μ to 20.25μ. Such heterogeneity in megakaryocyte morphology has been described earlier [6].
In ITP, the mean size of megakaryocyte was 407.56μm2 in our study. This is higher than that found by Ohshima whose mean size was 239.4μm2 [4]. There was also not much of variation in the size of megakaryocytes in the present study with dimen-sions ranging from 19 to 25μ. The megakaryocytes in ITP were significantly larger than that in the control. Although both CML & ITP both show megakaryocyte hyperplasia, there is a difference in morphology and size which is related to the pathogenesis of the disease, being neoplastic in the former and reactive in the latter.
Another aspect of the study was to compare the megakaryocyte morphology with the extent of fibrosis. In the normal marrow and in ITP only one case showed a mild increase of fibrous tissue. There was however a striking difference in the fibrous tissue in the cases of CML. Six cases showed low grade fibrosis while the remaining nine showed higher grade of fibrosis with two showing collagenization of the marrow.
The mean size of megakaryocyte in low-grade fibrosis was 120μm2, which was significantly less than that in high-grade fibro-sis (201μ2). The megakaryocytes were also clustered and in sheets in CML. Many of the megakaryocytes were located in the paratrabecular area. The topographic disorganisation and heterogeneity in size is related to fibrosis and hence the prognosis varies in different cases [11].
The present study has examined a simple method of classifying megakaryocytes using morphometry in differrent disorders affecting megakaryocyte lineage.
Conclusion
Morphology of megakaryocytes is different in different disorders affecting megakaryocyte lineage. Morphometry of megakaryocyte is a simple tool that could be utilised in differentiating these disorders if the need arises.
References
- Thiele J, Kvasnicka HM, Fischer R. Bone marrow histopathology in chronic myeloid leukemia-evaluation of distinctive features with clinical impact. Histolpathol 1999; 14: 1241-1256
- Thiele J. Impact of clinical and morphological variables in classification and regression tree based survival analysis of CML with special emphasis on dynamic features. Eur J Haematol 1998; 60: 35-46
- Thiele J, Kvasnicka HM, Fischer R. Histochemistry and morphometry on bone marrow biopsies in chronic myeloproliferative disorders-aids to diagnosis and classification. Ann Hema-tol. 1999; 78: 495-506.
- Ohshima K, Kikuchi M, Takeshita M. A megakaryocytic analysis of the bone marrow in patients with myelodysplastic syndrome, myeloproliferative disorder and allied disorders. J Pathol 1995; 177: 181-189
- Thiele J, Kvasnicka HM, Diehl V et al Clinicopathological diagnosis and differential criteria of thrombocythemia in various myeloproliferative disorders by histopathology, histo-chemistry and immunostaining from bone marrow biopsies. Leukemia and Lymphoma 1999; 33: 207-218
- Burkhardt R, Bartl R, Jager K et al. Working classification of chronic myeloproliferative disorders based on histological, hematological and clinical findings. J Clin Pathol 1986; 39: 237-252.
- Burkhardt R, Bartl R, Beil E et al. Myelofibrosis-osteomyelo-sclerosis syndrome- review of literature and histomorphology. Adv Biosciences 1975;16: 9-56
- Georgii A, Vykoupil KF, Thiele J. Chronic megakaryocytic granulocytic myelosis-CMGM. A subtype of chronic myeloid leukemia. Virchow Arch (Pathol Anat) 1980; 389: 253-268
- Thiele J, Funke S, Holgado S, Choritz H, Georgii A. Megakaryopoiesis in chronic myeloproliferative diseases. A morphometric evaluation with special emphasis on primary thrombocythemia. Anal Quant Cytol 1984; 6:155-167
- Bartl R, Frisch B, Wilmanns W. Potential of bone marrow biopsy in chronic myeloproliferative disorders. Eur J Haematol 1993; 50: 41-52
- Khonglah Y, Basu D, Dutta TK. Bone marrow trephine biopsy findings in chronic myeloid leukemia. Malaysian J Pathol 2002; 24: 37-43
- Nafe R, Georgii A, Kaloutsi V et al Planimetric analysis of megakaryocytes in the four main groups of chronic myeloproliferative disorders. Virchows Arch (Cell Pathol Incl Mol Pathol) 1991; 61: 111-116.