- Biomedical Research (2012) Volume 23, Issue 4
Mean Platelet Volume in Assessment of Brucellosis Disease
Zeynel Abidin Öztürk1, Hakan Sayıner2, Mehmet Emin Kuyumcu3, Yusuf Yesil3, Esen Savas4, Zeynel Abidin Sayıner4, Bünyamin Kısacık5, Yalçın Kepekçi11Gaziantep University, Faculty of Medicine, Department of Internal Medicine, Division of Geriatric Medicine, 27100 Sahinbey, Gaziantep, Turkey
2Adıyaman University, Faculty of Medicine, Department of Infectious Disease, 02400 Adıyaman, Turkey
3Hacettepe University, Faculty of Medicine, Department of Internal Medicine, Division of Geriatric Medicine, 06100 Sihhiye, Ankara, Turkey
4Gaziantep University Faculty of Medicine, Department of Internal Medicine, 27100 Sahinbey, Gaziantep, Turkey
5Gaziantep University Faculty of Medicine, Department of Internal Medicine, Division of Rheumatology, 27100 Sahinbey, Gaziantep, Turkey
- Corresponding Author:
- Zeynel Abidin Öztürk
Gaziantep University
Faculty of Medicine
Department of Internal Medicine
Division of Geriatric Medicine
27100 Sahinbey, Gaziantep, Turkey
Accepted date: August 21 2012
Abstract
Brucellosis is a multisystemic infectious disease with high morbidity rates. Despite improvement in laboratory techniques diagnosis and monitoring response to treatment may be difficult in brucellosis in endemic regions. Mean Platelet Volume (MPV) is an index of platelet activation and mentioned to be influenced by inflammation. This study was undertaken to compare MPV with acute phase reactants in acute and posttreatment phase of brucellosis with healthy controls.A total of 39 brucellosis patients (male/female: 15/24) and 40 healthy controls (male/female: 23/17) were enrolled study. Brucellosis diagnosis was based on clinical, serological and bacteriological data. MPV and inflammatory markers were measured at the time of diagnosis and at the end of the treatment.MPV levels of patients in acute phase were significantly lower than control cases ( p < 0.001). Overall accuracy of MPV in determining acute brucellosis was 75.2% with a sensitivity, specificity, NPV and PPV of 74.4%, 75%, 75% and 74.4% respectively (AUC:0.774). We suggest that MPV might help in assessment of brucellosis as an inexpensive and easy applicable test with other inflammatory markers.
Keywords
mean platelet volume, inflammation, brucellosis
Introduction
Brucellosis is a well known multisystemic infectious disease widespread around the world and results in many different clinical features and complications [1,2].The mortality of the disease is low; however, morbidity rates are much higher [1]. The diagnosis of brucellosis include clinical features, serology and culture [3,4]. An inflammatory process occurs in brucellosis which causes increase in acute phase reactants [5,6]. However there are important problems for physicians in brucellosis endemic regions; firstly in diagnosis of some cases the laboratory findings are inadequate [3,6,7]. Secondly antibody levels may remain elevated after the end of treatment and it is difficult to decide to stop or continue medication [8]. For this reason the adjunctive use of additional markers may add significant benefit for diagnosis and following of disease.
Mean Platelet Volume (MPV), the most extensively studied platelet activation marker, is routinely measured by complete blood count analyzers. MPV has been shown as an inflammatory marker in some diseases including myocardial infarction, ischemic stroke ulcerative colitis, Crohn’s Disease, acute appendicitis, Crimean-Congo hemorrhagic fever (CCHF), pulmonary tuberculosis [9-15]. To date, there is no study reporting the role of MPV in human brucellosis and its correlation with other inflammatory (ESR-Erythrocyte Sedimentation Rate, CRP-C Reactive Protein) markers. The present study was undertaken in order to investigate MPV’s relation to traditional inflammation markers in the acute phase of brucellosis and at the end of the treatment and compared them with healthy subjects.
Subjects and Methods
Thirty-nine diagnosed and treated patients with brucellosis were enrolled into study. 40 age and sex-matched healthy volunteers formed control group. Brucellosis diagnosis was based on clinical (fever, arthralgia, sweating, malaise, hepatomegaly, splenomegaly signs of focal disease), serological and bacteriological data. The diagnostic criteria of brucellosis were isolation of a Brucella species from blood cultures and/or antibody titer of Brucella tube agglutination method ≥ 1/160 in association with compatible clinical findings. All patients were treated with combination of doxcycline and rifampicin or streptomycin for 6 weeks. Responders were identified as improvements of clinical findings with Brucella tube agglutination ≤ 1/80 titer after the treatment. All of patients adjusted to the treatment and completely recovered clinically at the end of the treatment. The control group’s Brucella tube agglutination tests were negative, ESR and CRP levels were normal and did not have any complaints. Platelet numbers, MPV were recorded at the time of diagnosis and at the end of the treatment.
All complete blood count (CBC) analysis was performed in hematology laboratory of our hospital. Two millilitres of blood were taken into standardized tubes containing 0.04 ml of the 7.5% K3 salt of ethylenediaminetetraacetic acid (EDTA) from each subject. MPV increases over time as platelet swell in EDTA, therefore optimal MPV measurement should be within 2 hours of blood sampling [16]. Our CBC analysis was performed with the same analyzer within 2 hours after collection of blood samples with the use of a Cell-Dyn 3700 SL analyzer (Abbott Diagnostics, Chicago, USA). Hematological parameters which consisted of hemoglobin (Hb, range 14-18 g/dL for men, 12- 16 g/L for women), white blood cell (WBC, range 4.1- 11.2 x109/L), platelet count (PLT, range 150-400 x109/L), MPV (range 6.9-10.8 fL) were analyzed by standard methods, with a time-to-result of approximately 5 min. Also blood samples were collected into tubes 3.2 % sodium citrate for ESR and 10-ml serum tube for CRP. ESR and CRP were determined using automatic devices. The threshold levels for ESR and CRP were 20 mm/h and 0- 4.9 mg/dL, respectively.
Patients with smoke, medication, pregnancy and abnormal result of renal and liver function tests, other infection diseases and inflammatory conditions were excluded from study.
Statistical Analysis
SPSS (Statistical Package for Social Sciences) for Windows 15.0 programme was used for statistical analysis.
All data were entered into a database and were verified by a second independent person. The variables were investigated using visual (histograms, probability plots) and analytical methods to determine whether or not they are normally distributed. Data are presented as mean and ±S.D. for normally distributed variables (Hb, WBC in acute phase, PLT, MPV) and as median± IQR (Interquartile Range) for skew distributed continuous variables (age, after treatment WBC, ESR, CRP). Categorical variables are shown as frequencies.
One-Sample T test for normally distributed and Wilcoxon test for not normally distributed variables were used to compare acute and after treatment phase of patients with Brucellosis. Independent Samples T test and Mann- Whitney U test were used to compare Brucella and control group patients. Correlations between parameters were evaluated with Pearson and Spearman correlation tests. Two-sided values of p < 0.05 were considered as statistically significant. MPV, ESR, CRP,PLT and WBC values in predicting inflammation of acute phase of brucellosis were analyzed using Receiver Operating Characteristics (ROC) curve analysis. When a significant cut-off value was observed, the sensitivity, spesificity, positive and negative predictive values (PPV, NPV) were presented. While evaluating the area under the curve, a 5% type-1 error level was used to accept a statistically significant predictive value of the test variables. Two-sided values of p < 0.05 were considered as statistically significant.
Results
Thirty-nine brucellosis patients 15 male, 24 female; median age and IQR 28, (19-42) years and 40 control patients 23 male, 17 female; median age and IQR 29.5, (25.25-34.50) years were enrolled in this study.
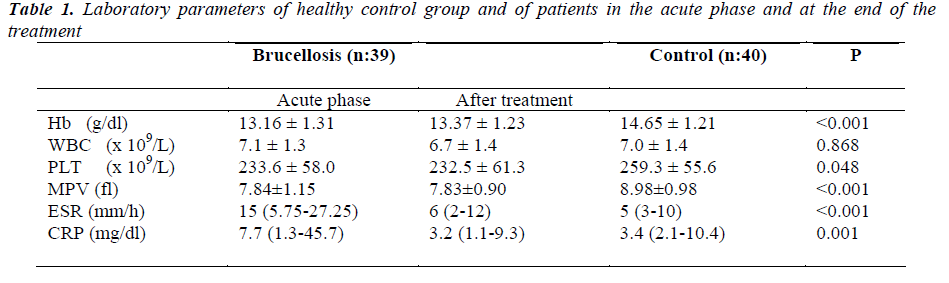
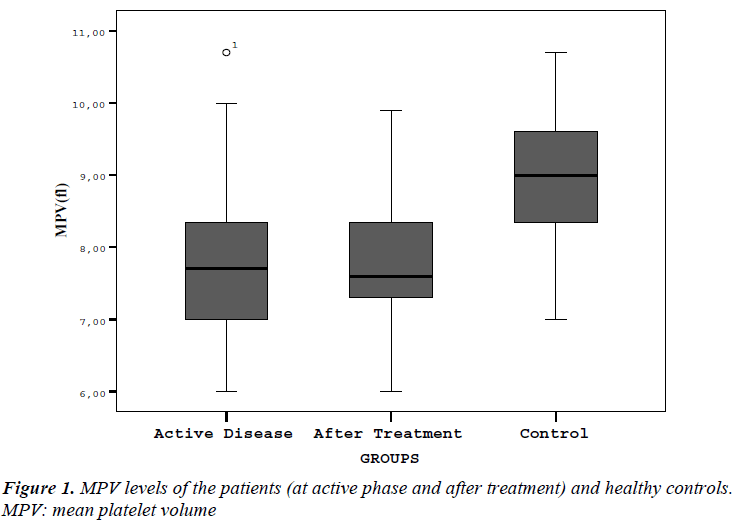
Laboratory parameters of patients and control group are presented in Table I. Hb, PLT, MPV, ESR and CRP values in acute phase of patients were statistically different from control patients. Figure 1 shows mean MPV values of brucellosis patients on acute and after treatment phase of the disease compared with controls. There were no difference in Hb, WBC, PLT and MPV levels between acute and after treatment phase of brucellosis cases. ESR and CRP levels in acute phase were significantly higher than posttreatment results (respectively, p:<0.001,- <0.001).
CRP was positively correlated with acute phase Hb (r: 0.230, p:0.042). A negative correlation was found between ESR and MPV values (r:-0.264, p:0.020) in acute brucellosis patients.
After treatment Hb, PLT, MPV and CRP values were still statistically different from control cases (respectively, p<0.001, 0.045, <0.001 and <0.001).
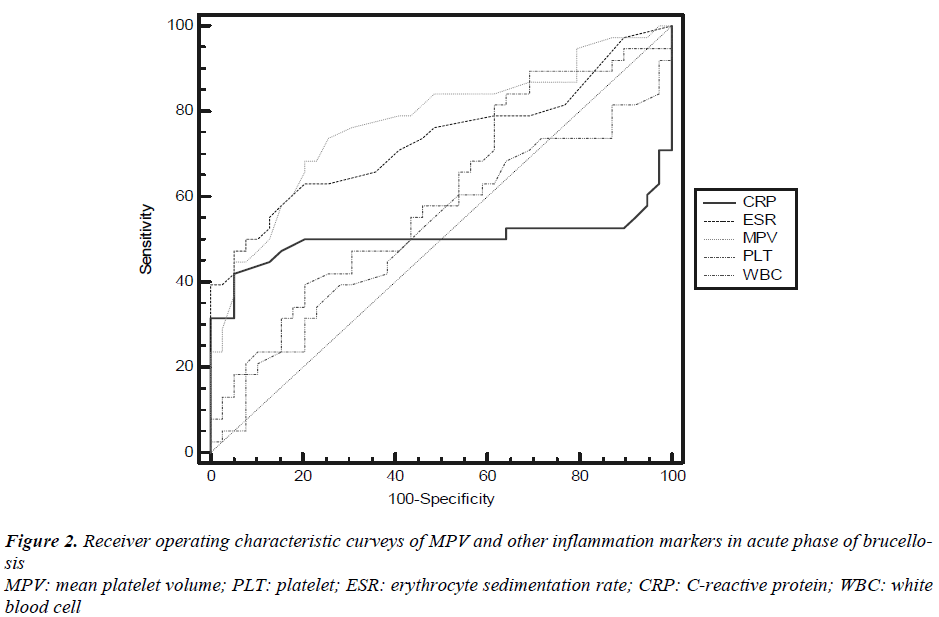
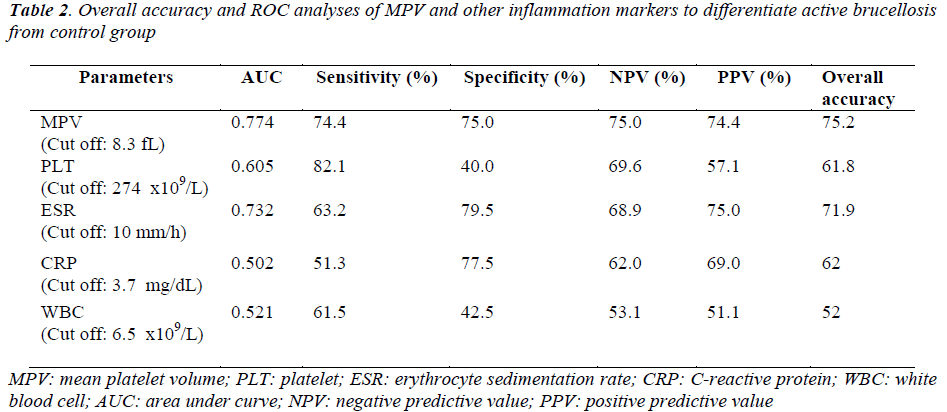
The receiver operating characteristic (ROC) curveys of MPV and other inflammation markers for diagnosis of active brucellosis were shown in Figure 2. ROC curve analysis suggested that the optimum MPV level cut-off points for acute brucellosis was 8.3 fL, with a sensitivity specificity, NPV and PPV of 74.4%, 75%, 75% and 74.4% respectively (Table 2). In determining acute disease MPV had highest and WBC had lowest overall accuracy percent.
Discussion
In this study it was revealed that MPV levels were lower in acute brucellosis cases when compared with controls. MPV levels remain low even the treatment completed successfully.
Brucellosis is a zoonotic systemic inflammatory disease, particularly in Mediterranean regions. Clinical manifestations include fever, night sweats, anorexia, arthralgia, weight loss and depression [1,4,17]. The human host response to the bacteria is determined via Th1-mediated immunity which is primarly managed by activated macrophages [18,19]. Interferon-gamma (IFN-γ) not only has a central role in the pathogenesis, it also manages controlling brucellosis by activating macrophages and inducing a bactericidal effect by natural killer cells and T lymphocytes.[20] Previous studies reported that IFN-γ levels were significantly higher in patients with brucellosis than control group [5,20]. A number of cytokines, such as tumor necrosis factor-alpha (TNF-α), IFN-γ, interleukin-1 and 12 (IL-1, IL-12) and other inflammatory mediators neopterin, chitotriosidase are also secreted by activated macrophages [18,21]. Although laboratory tools for diagnosis of brucellosis include culture and serology, there are also some difficulties in diagnosis and monitoring therapy success. The role of platelets in brucellosis pathophysiology has not been illustrated yet. In this setting the main aim of our study was to assess MPV in acute phase and posttreatment phase of brucellosis with control group.
Acute phase reactants; CRP and ESR increase in acute brucellosis cases and decrease to normal levels after treatment. CRP is a good marker in the diagnosis and in monitoring the efficiency of the treatment [6,22,23]. However in endemic areas it can be difficult to classify acute, chronic and recurrent cases of brucellosis.
MPV, the most commonly used measure of platelet size is a simple marker of platelet function and activation.[24]. It may be measured by CBC analyzers with no additional cost and is influenced by inflammation [24]. Increased MPV levels have been reported in conditions such as metabolic syndrome, myocardial infarction, atrial fibrillation, acute ischemic stroke, Alzheimer’s disease, diabetes mellitus and acute exacerbation of chronic obstructive pulmonary disease [9,10,13,25-30]. High MPV levels were also determined in infectious diseases such as pulmonary tuberculosis, CCHF and hydatid cyst disease [12,31,32]. On the other hand some recent studies have investigated lower MPV levels in active inflammatory bowel disease, rheumatic arthritis, ankylosing spondylitis, acute pancreatitis and appendicitis [15,33-35]. Based on these conflicting studies, it seems that both high and low MPV levels have a diagnostic and prognostic value for different inflammatory diseases.
To our knowledge this is the first study depicting decreased MPV levels in brucellosis when compared with healthy subjects. Overall accuracy of MPV in predicting disease was superior compared with other inflammation markers. The correlation of MPV with ESR is also a remarkable point. The main reason of decreased MPV in brucellosis is unclear, but during inflammatory process IL-1, IL-6, trombopoetin and cytokines play important role in regulating megakaryocyte ploidy and platelet number [36-38]. Demirbag et al. reported TNF-α and IFN-γ levels were higher in acute phase of brucellosis than control group [5]. In Akbulut et al.’s study TNF-α and IL-6 levels were found to be higher in patients compared the values of posttreatment and the control group [39]. Among from these mediators, IL-6 is thought to be the major responsible factor for low MPV levels [33]. Another possible mechanism is considered as; larger platelets which are metabolically and enzmatically more active used in inflammatory process and smaller platelets cause a decrease of MPV [40].
In conclusion our results suggest that MPV is involved in the pathophysiology of brucellosis and could be a promising and easily available biomarker in diagnosis with a low cost. It should not be considered a stand-alone test for this use due to nonspecificity with other diseases. Although this is the first report in this topic, further studies with higher number of cases are needed to establish the relation between platelet indices and brucellosis.
References
- Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med 2005; 352: 2325-2336.
- Corbel MJ. Brucellosis: an overview. Emerg Infect Dis 1997; 3: 213-221.
- Ariza J. [Current diagnosis of brucellosis]. Med Clin (Barc) 1992; 98: 494-496.
- Young EJ. An overview of human brucellosis. Clin Infect Dis 1995; 21:283-9; quiz 90.
- Demirdag K, Ozden M, Kalkan A, Godekmerdan A, Sirri Kilic S. Serum cytokine levels in patients with acute brucellosis and their relation to the traditional inflammatory markers. FEMS Immunol Med Microbiol 2003; 39: 149-153.
- Dabdoob WA, Abdulla ZA. A panel of eight tests in the serodiagnosis and immunological evaluation of acute brucellosis. East Mediterr Health J 2000; 6: 304-312.
- Young EJ. Serologic diagnosis of human brucellosis: analysis of 214 cases by agglutination tests and review of the literature. Rev Infect Dis 1991; 13: 359-372.
- Ariza J, Pellicer T, Pallarés R, Foz A, Gudiol F. Specific antibody profile in human brucellosis. Clin Infect Dis 1992; 14: 131-140.
- Nadar SK, Blann AD, Kamath S, Beevers DG, Lip GY. Platelet indexes in relation to target organ damage in high-risk hypertensive patients: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). J Am Coll Cardiol 2004; 44: 415-422
- Papanas N, Symeonidis G, Maltezos E, et al. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets 2004; 15: 475-478.
- Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet 1991; 338: 1409-1411.
- Ekiz F, Gürbüz Y, Basar O, et al. Mean Platelet Volume in the Diagnosis and Prognosis of Crimean- Congo Hemorrhagic Fever. Clin Appl Thromb Hemost 2012.
- Sahin Balcik O, Bilen S, Ulusoy EK, et al. Thrombopoietin and Mean Platelet Volume in Patients With Ischemic Stroke. Clin Appl Thromb Hemost 2012
- Varol E, Uysal BA, Ozaydin M. Platelet indices in patients with pulmonary arterial hypertension. Clin Appl Thromb Hemost 2011; 17: E171-E174.
- Albayrak Y, Albayrak A, Albayrak F, et al. Mean platelet volume: a new predictor in confirming acute appendicitis diagnosis. Clin Appl Thromb Hemost 2011; 17: 362-366.
- Lancé MD, van Oerle R, Henskens YM, Marcus MA. Do we need time adjusted mean platelet volume measurements? Lab Hematol 2010; 16: 28-31.
- Sharda DC, Lubani M. A study of brucellosis in childhood. Clin Pediatr (Phila) 1986; 25: 492-495.
- Golding B, Scott DE, Scharf O, et al. Immunity and protection against Brucella abortus. Microbes Infect 2001; 3: 43-48.
- Hamerlinck FF. Neopterin: a review. Exp Dermatol 1999; 8: 167-176.
- Ahmed K, Al-Matrouk KA, Martinez G, Oishi K, Rotimi VO, Nagatake T. Increased serum levels of interferon-gamma and interleukin-12 during human brucellosis. Am J Trop Med Hyg 1999; 61: 425-427.
- Coskun O, Oter S, Yaman H, Kilic S, Kurt I, Eyigun CP. Evaluating the validity of serum neopterin and chitotriosidase levels in follow-up brucellosis patients. Intern Med 2010; 49: 1111-1118.
- Al-Kassab AS, Nur MA, Malik JM. Evaluation of serum C-reactive protein in the diagnosis of arthritic and non-arthritic brucellosis. J Trop Med Hyg 1991; 94: 92-96.
- Navarro JM, Mendoza J, Leiva J, Rodríguez-Contreras R, de la Rosa M. C-reactive protein as a prognostic indicator in acute brucellosis. Diagn Microbiol Infect Dis 1990; 13: 269-270.
- Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis 1996; 7: 157-161.
- Yesil Y, Kuyumcu ME, Cankurtaran M, et al. Increased mean platelet volume (MPV) indicating the vascular risk in Alzheimer's disease (AD). Arch Gerontol Geriatr 2011.
- Mayda-Domaç F, Misirli H, Yilmaz M. Prognostic role of mean platelet volume and platelet count in ischemic and hemorrhagic stroke. J Stroke Cerebrovasc Dis 2010; 19: 66-72.
- Coban E, Yazicioglu G, Berkant Avci A, Akcit F. The mean platelet volume in patients with essential and white coat hypertension. Platelets 2005; 16: 435-438.
- Martin JF, Bath PM, Burr ML. Mean platelet volume and myocardial infarction. Lancet 1992; 339: 1000- 1001.
- Yuce M, Cakici M, Davutoglu V, et al. Relationship between mean platelet volume and atrial thrombus in patients with atrial fibrillation. Blood Coagul Fibrinolysis 2010; 21: 722-725.
- Ulasli SS, Ozyurek BA, Yilmaz EB, Ulubay G. Mean platelet volume: an inflammatory marker in acute exacerbation of chronic obstructive pulmonary disease. Pol Arch Med Wewn 2012
- Tozkoparan E, Deniz O, Ucar E, Bilgic H, Ekiz K. Changes in platelet count and indices in pulmonary tuberculosis. Clin Chem Lab Med 2007; 45:1009-1013.
- Küçükbayrak A, Oz G, Fındık G, et al. Evaluation of platelet parameters in patients with pulmonary hydatid cyst. Mediterr J Hematol Infect Dis 2010; 2: e2010006.
- Yüksel O, Helvaci K, Basar O, et al. An overlooked indicator of disease activity in ulcerative colitis: mean platelet volume. Platelets 2009; 20: 277-281.
- Kisacik B, Tufan A, Kalyoncu U, et al. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine 2008; 75: 291-294.
- Beyazit Y, Sayilir A, Torun S, et al. Mean platelet volume as an indicator of disease severity in patients with acute pancreatitis. Clin Res Hepatol Gastroenterol 2011.
- Senaran H, Ileri M, Altinbas A, et al. Thrombopoietin and mean platelet volume in coronary artery disease. Clin Cardiol 2001; 24: 405-408.
- Martin JF, Trowbridge EA, Salmon G, Plumb J. The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res 1983; 32: 443-460.
- Brown AS, Hong Y, de Belder A, et al. Megakaryocyte ploidy and platelet changes in human diabetes and atherosclerosis. Arterioscler Thromb Vasc Biol 1997; 17: 802-807.
- Akbulut H, Celik I, Akbulut A. Cytokine levels in patients with brucellosis and their relations with the treatment. Indian J Med Microbiol 2007; 25: 387-390.
- Karpatkin S. Heterogeneity of human platelets. II. Functional evidence suggestive of young and old platelets. J Clin Invest 1969; 48: 1083-1087.