Research Article - Gynecology and Reproductive Endocrinology (2017) Volume 1, Issue 1
Maternal morbidity with early onset hypertensive disorders during pregnancy
Chhabra S* and Singh ADepartment of Obstetrics Gynaecology, Mahatma Gandhi Institute of Medical Sciences, Sewagram, Wardha, Maharashtra
- *Corresponding Author:
- Chhabra S
Department of Obstetrics and Gynecology
Mahatma Gandhi Institute of Medical Sciences
Sewagram, OSD, Dr Sushila Nayar Hospital Utavali
Melghat, Amravati, Maharashtra
E-mail: chhabra_s@rediffmail.com
Accepted date: November 20, 2017
Citation: Chhabra S, Singh A. Maternal morbidity with early onset hypertensive disorders during pregnancy. Gynecol Gest Surr - UK.2017;1(1):21-27
DOI: 10.35841/2591-7994.1.1.24-30
Visit for more related articles at Gynecology and Reproductive EndocrinologyAbstract
Background: Hypertensive disorders of pregnancy (HDsP) are being divided into early onset (EO), late onset (LO) disorders, depending upon gestation at diagnosis, because of differences in causes, pathology, outcome and sequale. It seems EO HDsP cause much more morbidity in the mother. Objective: To know differences in maternal morbidity EO HDsP compared to late onset disorders. Material Method: Study was done to know complications in cases of 1046 cases of EO LO HDsP, managed over 2 years, 11.73% of 8920 births during the study period. EO cases were (Category A)-20 to <28 weeks, (B) ? 28-<34 weeks and LO, (C) ? 34-<37 weeks, (D) ? 37 weeks. Results: Significantly more women with EO HDsP were primigravida of younger age. Significantly more women with very EO HDsP (Category A) had liver dysfunction compared to Category B, C, D, 85% had mild preeclampsia, 60% severe in A, 32% mild PE 27% severe PE, in LO cases, 9.58% mild PE, 50% severe PE in C, 19.56% mild PE in D, 44.11% severe PE. HELLP (Hemolysis, Elevated Liver enzymes and Low Platelet count) as well as renal failure were also more often diagnosed in EO cases. In LO no patient had grade III hypertensive retinopathy. Placental abruption occurred in 25% A, 14.66% B, 3.79% C, 2.67% D. There was no maternal death with individualized management. Conclusion: Younger women had EO disorders which were more often severe. There were more chances of multiorgan disorders in women with EO disorders compared to LO.
Keywords
Early disorders of pregnancy, Maternal complications.
Introduction
Hypertensive disorders of pregnancy (HDsP), a group of multiorgan disorders that significantly contribute to maternal, fetal/ neonatal morbidity and mortality are the second most common cause of maternal deaths worldwide [1-11]. Berg and colleagues reported that 16% of 3201 maternal deaths due to complications of HDsP between 1991 to 1997 in the United States also [12]. Smith et al. reported that preeclampsia complicated 3-8% of pregnancies in Western countries, and was a significant cause of maternal deaths, causing 10-15% of maternal deaths [13]. However some mothers with HDsP did well, others suffered a lot and this seemed to be dependent on the gestation at which disorder occurred [14]. For better understanding of the causes, course, and outcome, disorders are being divided as early onset (EO) and late onset (LO) disorders, depending upon gestation at which they are diagnosed. EO and LO HDsP are believed to be different disease entities with overlapping clinical signs [11,15- 18]. HDsP lead to many problems in the mother and baby, more so EO with commonalities but a lot is still not known. In resource poor settings the problems are more because of delay in diagnosis and delay in the appropriate management and research continues.
Objectives
Present study of women diagnosed with HDsP before 34 weeks (early) and after 34 weeks (late) was done to know differences in the demography, maternal morbidity, mortality in EO and LO HDsP.
Material Methods
Prospective study was carried out over 2 years in Obstetrics Gynaecology with the help of Biochemistry at a rural referral hospital after approval of the institute’s ethics committee and consent was taken from the study subjects. Study subjects were pregnant women or women in labour beyond 20 weeks with singleton pregnancy hypertension, with gestational hypertension (GH)/preeclampsia/eclampsia. Women, with pre-pregnancy hypertension diabetes/renal, liver disease, or other medical disorders, past or current smokers, not planning to deliver at the place of study were excluded. Investigations, including coagulation, liver, renal function and Doppler studies were done as was the practice. Women were divided in two major groups 20 weeks to less than 34 weeks (EO HDP) and 34 weeks onwards (LO HDsP). For better information, EO were further divided into group A, ≥ 20 to<28, B ≥ 28 to<34 and LO into C, ≥ 34 to<37 and D, ≥ 37. Information was collected with the help of a predesigned tool. Analysis was done for differences in complications in the mothers.
During the study period there were 16807 obstetric admissions, 1769 (10.53%) cases irrespective whether new or old were diagnosed to be having HDsP blood pressure diastolic 140 or more diastolic blood pressure 90 mm Hg or more and urine proteinuria may or may not have been present. But 40.87% women either did not fit into inclusion criteria or did not plan to deliver at the place of study, so 1046 women became the study subjects, 11.73% of 8920 births during the study period. Eighty cases were of Category A, 0.89% of all births, 2.31% births of gestation similar to Category A and 7.65% of all births with HDsP cases, 191 were of Category B, 2.14% of all births, 2.88% births of gestation similar to Category B, 18.26% of births in HDsP cases, 475 patients were of Category C, 5.33% of all births, 15.25% of births with gestation similar to the Category C and 45.41% of all cases with HDsP and 300 were of Category D, 3.37% of all births, 6.42% births of gestation similar to Category D and 28.68% of all cases of HDsP.
Results
All 4 cases, in Category A 58.75% (47 of 80) women were primigravida, in Category B 83.24% (159 of 191) primigravida. In Category C, 85.05% (404 of 475) and in Category D 73% (219 of 300) primigravida.
In EOHDsP cases, more women were younger (20-24 years), 60% (48 of 80) of Category A and 52.88% (101 of 191) of Category B, compared to 33.47% (159 of 475) of Category C, 23.33% (70 of 300) of D, significant difference (P value<0.01). Of 80 women of Category A, 17 (21.25%) were multigravida, only one woman (5.88%) had history of HDsP in past pregnancy and of 191 women of Category B 32 (16.75%) were multigravida and 12.5% (4 of 32) of them had HDsP in previous pregnancy. Of 475 women in Category C 73 (15.37%) was multigravida and 19.17% (14 of 73) of them had HDsP in past pregnancy.
Of 300 women in Category D, 82 (27.33%) were multigravida and 14.63% (12 of 82) had HDsP in past pregnancy. In very EO cases, significantly fewer women had HDsP in past pregnancy (Table 1). Significantly more women with EO had severe disease than LO cases.
| Category | Hypertensive disorders of pregnancy | ≤ 19 yrs n=41 |
20-24 yrs n=384 |
25-29 yrs n=526 |
≥ 30 yrs n=98 |
Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| P0 | P1-P2 | P0 | P1-P2 | P0 | P1-P2 | P0 | P1-P2 | |||
| A n=80 |
Mild PIH | 1 | - | 6 | 3 | - | - | - | - | 10 |
| Severe PIH | 5 | - | 15 | 3 | 7 | 4 | 1 | - | 35 | |
| Mild PE | - | - | 7 | 3 | 4 | - | - | - | 14 | |
| Severe PE | 1 | 1 | 10 | 2 | 4 | 2 | - | - | 20 | |
| Eclampsia | - | - | 1 | - | - | - | - | - | 1 | |
| B n=191 |
Mild PIH | 2 | - | 10 | 3 | 2 | 2 | 1 | - | 20 |
| Severe PIH | 2 | - | 34 | 6 | 27 | 6 | 4 | 1 | 80 | |
| Mild PE | 1 | - | 17 | 1 | 14 | 2 | 2 | - | 37 | |
| Severe PE | 1 | - | 21 | 6 | 11 | 2 | 4 | 3 | 48 | |
| Eclampsia | - | - | 4 | - | 2 | - | - | - | 6 | |
| C n=475 |
Mild PIH | 7 | - | 60 | 14 | 74 | 8 | 2 | 5 | 170 |
| Severe PIH | 4 | - | 20 | 3 | 49 | 4 | 1 | 2 | 83 | |
| Mild PE | 6 | - | 30 | 3 | 79 | 18 | 10 | - | 146 | |
| Severe PE | 1 | - | 18 | 2 | 11 | 2 | 7 | 5 | 46 | |
| Eclampsia | - | - | 8 | 1 | 15 | - | 2 | 4 | 30 | |
| D n=300 |
Mild PIH | 5 | - | 28 | 12 | 52 | 18 | 17 | - | 132 |
| Severe PIH | 1 | - | 6 | 2 | 15 | 10 | 1 | 1 | 36 | |
| Mild PE | 3 | - | 8 | 2 | 42 | 21 | 10 | 6 | 92 | |
| Severe PE | - | - | 9 | 1 | 10 | 7 | 7 | - | 34 | |
| Eclampsia | - | - | 2 | - | 2 | - | 1 | 1 | 6 | |
| TOTAL | 40 | 1 | 314 | 67 | 420 | 106 | 70 | 28 | 1046 | |
Table 1: Age and parity and EO/LO hypertensive disorders.
Significantly more cases of second trimester HDsP had liver dysfunction compared with Category B, C and D. In Category A, 85% (12 of 14) of mild PE and 60% (12 of 20) of severe PE, in Category B, 32% (12 of 37) with mild preeclampsia and 27% (13 of 48) of severe PE, while in LO cases in Category C, 9.58% (14 of 146) of mild PE, 50% (23 of 46) of severe PE and in Category D 19.56% (18 of 92) of mild PE and 44.11% (15 of 34) of severe PE had deranged liver enzymes (Table 2).
| Severity of Hypertensive Disorders of Pregnancy | Total | ||||||
|---|---|---|---|---|---|---|---|
| Category | Mild PIH | Severe PIH | Mild PE | Severe PE | Eclampsia | ||
| A n=80 |
N | 10 | 35 | 14 | 20 | 1 | 80 |
| % in category | 12.5 | 43.75 | 17.5 | 25 | 1.25 | 7.65 | |
| % of HDP | 3.0 | 14.9 | 4.8 | 13.5 | 2.3 | 100.0 | |
| B n=191 |
n | 20 | 80 | 37 | 48 | 6 | 191 |
| % in category | 10.4 | 41.9 | 19.4 | 25.1 | 3.1 | 18.26 | |
| % of HDP | 6.0 | 34.1 | 12.8 | 32.4 | 13.9 | 100.0 | |
| C n=475 |
N | 170 | 83 | 146 | 46 | 30 | 475 |
| % in category | 35.8 | 17.5 | 30.7 | 9.6 | 6.3 | 45.41 | |
| % of HDP | 51.2 | 34.5 | 50.5 | 31.1 | 69.7 | 100.0 | |
| D n=300 |
n | 132 | 36 | 92 | 34 | 6 | 300 |
| % in category | 44.0 | 12.0 | 30.6 | 11.3 | 2.0 | 28.68 | |
| % of HDP | 39.8 | 15.4 | 31.8 | 22.9 | 13.9 | 100.0 | |
| Total | N | 332 | 234 | 289 | 148 | 43 | 1046 |
| % | 31.7 | 22.4 | 27.6 | 14.1 | 4.1 | 100 | |
Table 2: Severity of hypertensive disorders of pregnancy in early onset and late onset disorders.
In EO cases, in Category A (80) 8.75%, and in Category B (191), 10.47% had Haemolysis, Elevated liver enzymes, Low Platelets (HELLP). In late onset cases, in C (475), 7.37% and in D (300), 6.0% had HELLP. HELLP was more often diagnosed in EO cases but difference was not significant (p ≥ 0.05). Overall HELLP complicated 7.65% study cases, (9.96% mild PIH, 11.30% severe PIH, 17.65% severe PE and 3.92% mild PE, 0.73% eclampsia in EO cases and 6.84% in LO cases, 9.33% in severe PIH, 30.0% in severe PE and 2.52% in mild PE, 0.39% in eclampsia in LO cases.
Of 80 women of Category A, 8 (10%) had grade I, 3 (3.75%) grade II and 3 (3.75%) had grade III hypertensive retinopathy. Of 191 women of Category B, 6 (3.14%) had grade I, 3 (1.57%) grade II and one (0.52%) had grade III hypertensive retinopathy. In LO HDsP 0.63% (3 of 475) patients in C and 0.67% (2 of 300) in Category D had grade I, one (0.21%) in C had grade II hypertensive retinopathy. No patient in LO cases had grade III hypertensive retinopathy.
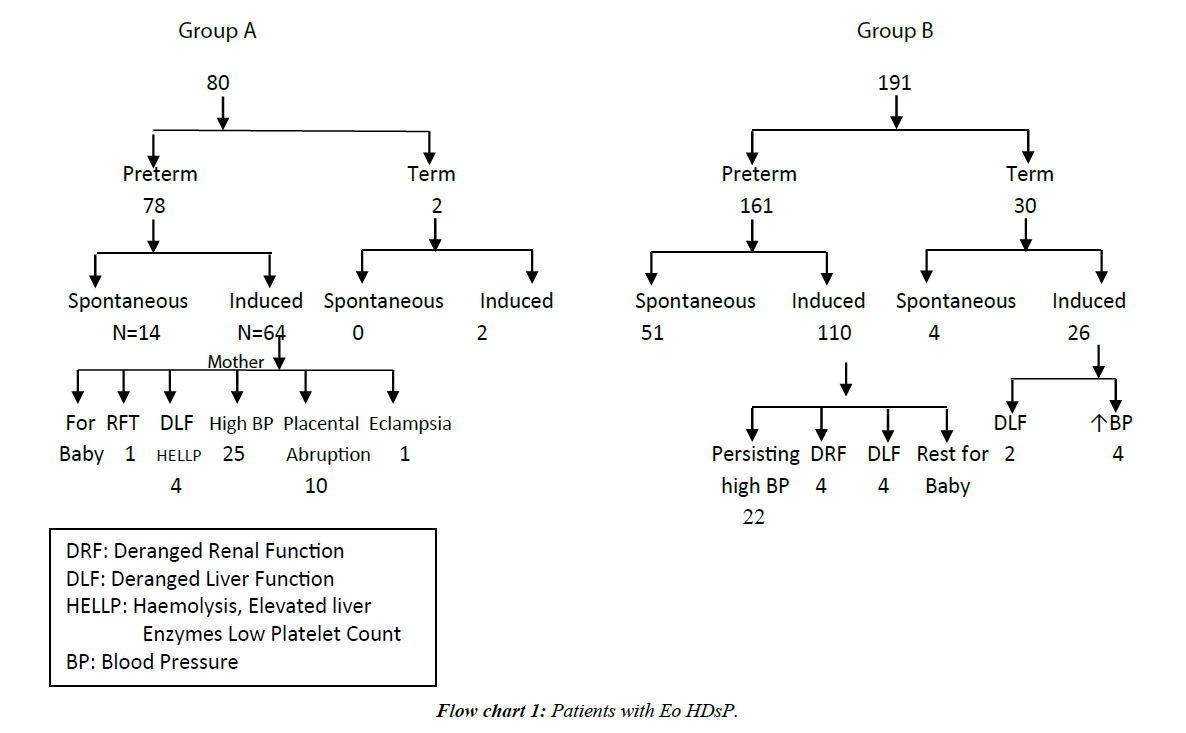
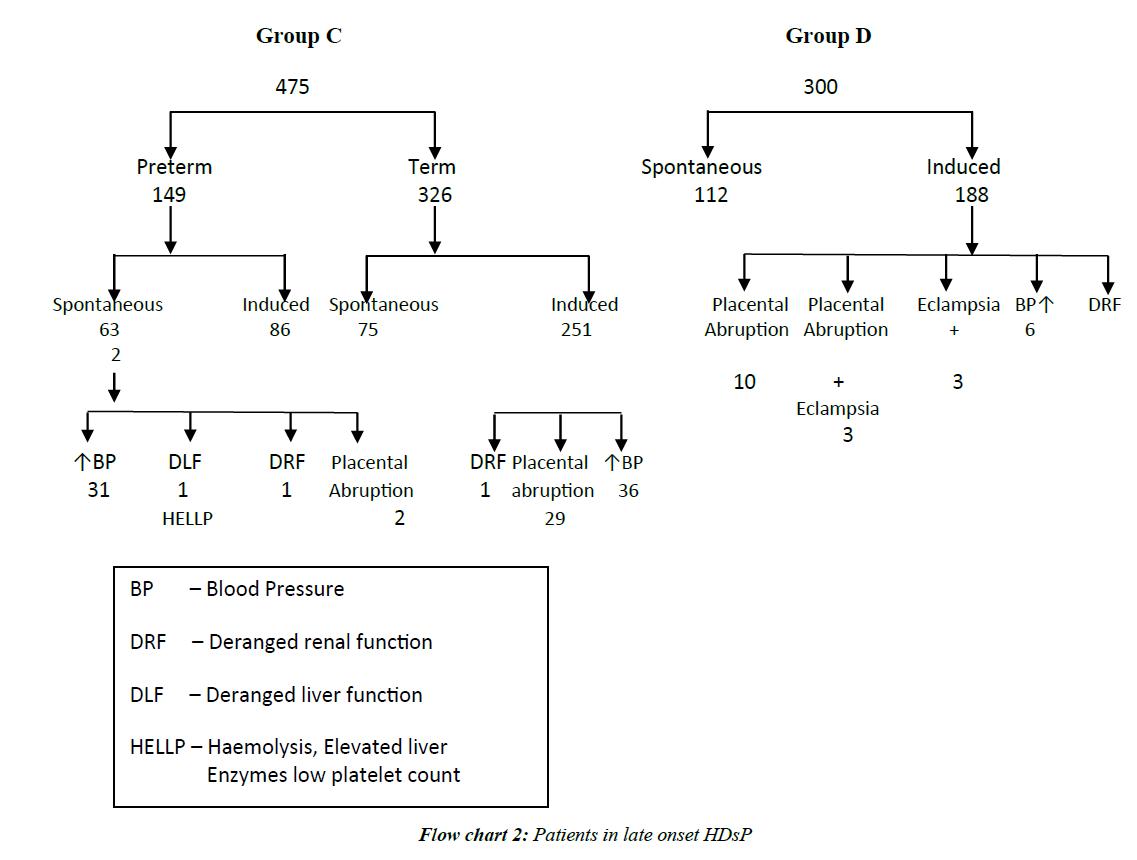
In second trimester HDsP, (A) 1.25% patients developed acute renal failure at 32 weeks, one patient (1.25%) with severe PE had eclampsia at 33 weeks and 17.50% women had placental abruption. In Category B, 1.05% had acute renal failure at ≥ 28-32 weeks, 2 patients (1.05%) of severe PE had eclampsia at ≥ 32-34 weeks and 12.04% patients had placental abruption. More cases of Category A and B had placental abruption (25% in Category A and 14.66% in Category B 3.67% in Category C, 2.67% in Category). In Category C 0.21% patients had acute renal failure at 36 weeks, and 3.79% patients had placental abruption. In Category D, 0.66% had acute renal failure at 38 weeks and 2.67% (8 of 300) patients had placental abruption. Renal failure occurred in less often in LO cases (Flow charts 1 and 2).
In Category A, 1.25%, in Category B, 1.05%, in Category C, 0.21% and in Category D, 0.33% had intra-operative couvlaire uterus. In Category A, 2.5% (2 of 80), in Category B, 3.14% (6 of 191) in Category C, 0.42% (2 of 475) and in Category D, 0.66% (2 of 300) patients had post-partum hemorrhage. Couvlaire uterus and PPH occurred significantly more often in EO cases. In Category B, 0.52% (1 of 191) had haematuria also (Table 3 and Flow chart 1).
| Category | Couvlaire uterus | Oligouria | Haematuria | Presented as Eclampsia | Developed later Eclampsia | Total | % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |
| A n=80 |
1 | 1.25 | 1 | 1.25 | 0 | 0.0 | 1 | 1.25 | 1 | 1.25 | 4 | 5 |
| B n=191 |
2 | 1.05 | 2 | 1.05 | 1 | 0.52 | 6 | 3.14 | 2 | 1.05 | 13 | 6.8 |
| C n=475 |
1 | 0.21 | 1 | 0.21 | 0 | 0.0 | 30 | 6.32 | 0 | 0.0 | 32 | 6.73 |
| D n=300 |
1 | 0.33 | 2 | 0.66 | 0 | 0.0 | 6 | 0.0 | 0 | 0.0 | 9 | 3 |
| Total | 5 | 2.84 | 6 | 3.17 | 1 | 0.52 | 43 | 42.39 | 3 | 7.25 | 58 | 21.53 |
Table 3: Maternal morbidity during pregnancy, labour and post-partum In EO and LO HDsP.
There was no maternal death. No woman developed pulmonary or laryngeal edema or cerebral haemorrhage, but 1.25% cases of preeclampsia in Category A, 3.14% of Category B, developed eclampsia after admission. Only 2.5% (2 of 80) patients of A could reach term gestation.
In Category A, minimum latency (from diagnosis to birth) was 14 days, maximum 106 days and mean was 54 days. In Category B, minimum latency was 5 days, maximum 76 days and mean was 60 days. In Category C, minimum latency was one day, and maximum 38 days and mean was 10 days and in Category D, minimum latency was one day, maximum 21 days and mean was 14 days. Foetal neonatal outcome and mode of delivery have been analysed separately.
Discussion
The concept of EO and LO HDsP is recent, and it is being widely accepted that these are two entities. They have different etiologies and should be regarded as different forms of the disease [19-22]. The EO HDsP comprises a small subset of all cases of HDsP (5 to 20%), but is the most dangerous. Earlier Sibai et al. reported a gradual increase in the cases of HDsP with increasing gestation, 32% of all in the second trimester, 32% between 28 to 36 weeks, and 36% between 37 to 40 weeks [23]. Meis et al. reported 15.3% incidence of second trimester HDsP [24], Shenhav et al. and Onah et al. have reported incidence of 24.51% and 23.4%, respectively [25,26]. Similarly Yang et al. reported a gradual increase in incidence with gestation, 9.40% of all cases before 28 weeks, 19.61% between 28-31 weeks of gestation, 13.33% at 32-33 gestational weeks, and 57.66% with onset ≥ or =34 weeks of gestation [19]. Banhidy et al. reported that of 1017 patients of HDsP, 3.9% had developed hypertensive disorder in the 4th month of pregnancy 26.8% in 8th month and 24.7% in 9th month of pregnancy [27]. However if the high blood pressure is detected prior to 20 weeks, it is considered essential hypertension rather than HDsP. Lisonkova et al. reported increased incidence with increasing gestation; EO and LO preeclampsia rates 0.38% and 2.72% respectively [28]. In the present study of 8920 births, HDsP were, 0.89% in second trimester cases, 2.14% between ≥ 28 to <34 weeks, 5.33% of ≥ 34 to <37 weeks and 3.37% at term gestation. Ebeigbe et al. reported EO HDsP contributed to 6.3% of all cases of HDsP and most women presented between 28-32 weeks gestation (78.3%). The disease was severe at presentation or rapidly progressive in 39 cases (84.8%), leading to delivery within 72 hours of presentation. Most of the cases were delivered by CS (58.7%) [29]. the researchers concluded that most of EO cases were associated with significantly higher risk of obstetric interventions. However, Kenneth et al. reported that LO HDsP also presented in severe disorder [30]. In a retrospective study of 264 singleton pregnancies with LO disease, researchers reported that 57.6% were severe, with median gestational age at diagnosis 37 (34-43) weeks, 30.7% of patients experienced ≥ 1 major maternal complications and also 34 (12.9%) had eclampsia and concluded that LO HDP were not innocuous condition.
Crispi et al. reported that 83% EO and 41% LO cases were nulliparous [16] and Akolekar et al. reported 58% EO and 64.2% LO nulliparous [31]. Earlier in the studies by Eskenazi et al. and Coonrod et al. There was no difference in numbers of primigravida in EO and LO disorders [32,33]. In the present study 271 (25.9%) had EO HDsP (<34 weeks) and of them, 81.55% (221 of 271) were primigravida and of 775 patients of LO disorders, also 80.39% (623 of 775) were primigravida, no difference. However, primigravida were almost double than overall primigravida (42%) during the same period. In Category A, 10% (8 of 80), 3.14% ( 6 of 191) in B and in LO cases, in C, 3.79% (18 of 475 ) and in D, 3% (9 of 300) were teenage mothers, significantly more teen age cases in Category A as compared to Category B, C and D (p<0.05). The mean age in Category A was, 23.50 years, 23.96 years in B, 24.12 years in C and 24.12 years Category D. Women in A and B were significantly younger compared to Category D (p<0.001). Crispi et al. reported the mean age of EO cases 29 ± 5 years and LO disorders 32 ± 5 years [34]. However Valensise et al. reported 32±4 years mean age in EO cases and 34±4 years in LO cases [17]. Although an age more than 35 years is linked to a higher risk for preeclampsia, the importance of age in EO and LO preeclampsia needs further research. Also more studies are needed in view of differences in the age of marriage around the world.
In a study by Ebeigbe et al. the EO disease was severe at presentation or rapidly progressive in 84.8% cases as were the cases of EO disorder in the present study [29]. EO affects vital organs more often, more seriously than LO cases. Onah et al. reported 175 (23.4%) of the 749 cases of HDsP at less than 30 weeks gestational age (early) and the 574 were of 30 weeks onwards (late), with twofold increase in MMR and a significantly increased incidence of renal failure and HELLP syndrome in EO cases [26]. The researchers concluded that at admission prediction of the clinical course of the disease and the development of additional maternal complications was not possible. In the present study maternal mortality could be prevented with individualized management, though more women did have placental abruption, renal, hepatic and coagulation dysfunction in EO cases. But no one developed pulmonary oedema or obvious cerebral haemorrhage.
Murphy et al. reported 21% incidence of HELLP/ELLP in EO HDsP [35]. Onah et al. also reported significantly increased risk of HELLP in EO disorders [26]. The researchers reported that the high incidence of HELLP in their study, may be because, more cases were managed conservatively for longer duration. However this aspect needs to be researched as it may also be that those who have multi-organ disease at a very early stage have clinically obvious early disease. Sezik et al. reported 21.8% incidence of HELLP in EO disorders [36], similar to the present study, Gasem found incidence of HELLP syndrome 8.3% in EO HDsP [37]. In another study, Makinde et al. reported 8.3% incidence of HELLP in cases of severe PE in EO [38].
Murphy et al. in their study reported that, of 21% preterm preeclampsia cases. Thirteen percentages had renal failure, and 15% had placental abruption [35]. The study revealed that a conservative approach to the management of EO preeclampsia resulted in good obstetric outcome for the majority of fetuses, but this needed to be balanced against the significant risk of increase in morbidity to the mothers. Jenkins et al. reported that in 39 women with severe preeclampsia in second trimester (<25 weeks), 54% experienced morbidity like placental abruptio, HELLP, renal insufficiency, and eclampsia [39]. An EO case has severe placental pathophysiology. Yeo reported that LO preeclampsia as an autonomic dysregulation is a new approach [40]. Unlike EO preeclampsia, which involves severe placental pathophysiology and needs a lot of research, LO preeclampsia intact placenta with maternal cardiovascular dysregulation may be prevented with a lifestyle intervention, in particular, low intensity exercise. Yinon reported that women with a history of EO preeclampsia or foetal growth restriction without preeclampsia exhibited impaired vascular function, which might explain their predisposition disease and their higher risk of future vascular disease. In the present study, not only some women of EO developed eclampsia but more women had renal failure, placental abruption, couvlaire uterus and PPH. Bombrys et al. reported that 36% (4 of 11) patients of EO disorder (at ≥ 32 weeks) with expectant management had pulmonary edema or hemolytic anemia and concluded that because there was significant maternal morbidity at ≥ 32 weeks with minimal and neonatal benefit, consideration was needed to be given for delivery of cases following corticosteroid administration [41].
EO HDsP, more so second trimester HDsP have more severe presentation and cause more serious morbidities. EO HDsP has more chances of renal failure, placental abruption HELLP etc. Individualized management is essential. In future if certain markers are found, a lot can be done to prevent maternal morbidity. A lot of research is needed.
References
- NHBPEP. Report of the National high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol 2000;183:S1-S22.
- ACOG. Diagnosis and management of preeclampsia and eclampsia. ACOG Practice Bulletin Obstet Gynecol 2002;99:59-67.
- Magee LA, Von Dadelszen P, Bohun CM, et al. Serious perinatal complications of non-proteinuric hypertension: an international, multicentre, retrospective cohort study. J Obstet Gynaecol Can 2003;25:372-382.
- Cifkova R. Hypertension in pregnancy. Vnitr Lek 2006;52:263-270.
- Khan KS, Wojdyla D, Say L, et al. Who analysis of causes of maternal death: A systematic review. Lancet 2006;367:1066-1074.
- Hill K, Thomas K, Abouzahr C, et al. Estimates of maternal mortality worldwide between 1990 and 2005: An assessment of available data. Lancet, 2007;370:1311-1319.
- Cifkova R. Hypertension In Pregnancy. Cas Lek Cesk 2009;148:65-71.
- Hogan MC, Foreman KJ, Naghavi M, et al. Maternal mortality for 181 countries, 1980-2008: A systematic analysis of progress towards millennium development goal 5. Lancet 2010;375:1609-1623.
- Jabeen M, Yakoob MY, Imdad A, et al. Impact of interventions to prevent and manage preeclampsia and eclampsia on stillbirths. BMC Public Health 2011;13:11S3-S6.
- Ronsmans C, Campbell, O. (2011). Quantifying The Fall In Mortality Associated With Interventions Related To Hypertensive Diseases Of Pregnancy. BMC Public Health, 11 Suppl 3, S8.
- Junus K, Centlow M, Wikstrom AK, et al. Gene expression profiling of placentae from women with early and late-onset pre-eclampsia: Down-regulation of the angiogenesis-related genes acvrl1 and egfl7 in early-onset disease. Mol Hum Reprod 2012;18:146-155.
- Berg CJ, Chang J, Callaghan WM, et al. Pregnancy-related mortality in the united states, 1991-1997. Obstet Gynecol 2003;101:289-296.
- Smith GN, Pudwell J, Walker M, et al. Ten-year, thirty-year, and lifetime cardiovascular disease risk estimates following a pregnancy complicated by preeclampsia. J Obstet Gynaecel Can. 2012;34:830-835.
- Uzan J, Carbonnel M, Piconne O, et al. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag 2011;7:467-474.
- Vatten LJ, Skjaerven R. Is pre-eclampsia more than one disease? BJOG 2004;111:298-302.
- Crispi F, Dominguez C, Llurba E, et al. Placental angiogenic growth factors and uterine artery doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol 2006;195:201-207.
- Valensise H, Vasapollo B, Gagliardi G, et al. Early and late preeclampsia: Two different maternal hemodynamic states in the latent phase of the disease. Hyper 2008;52:873-880.
- Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv 2011;66:497-506.
- Yang Z, Li R, Shi LY, et al. Clinical delimitation and expectant management of early onset of severe pre-eclampsia. Zhonghua Fu Chan Ke Za Zhi 2005;40:302-305.
- Yang Z, Wang JL, Huang P, et al. Study on different onset patterns and perinatal outcomes in severe preeclampsia. Zhonghua Fu Chan Ke Za Zhi 2006;41:302-306.
- Huppertz B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008;51:970-975.
- Kang A, Struben H. Pre-eclampsia screening in first and second trimester. Ther Umsch 2008;65:663-666.
- Sibai BM, Mercer B, Sarinoglu C, et al. Severe preeclampsia in the second trimester: Recurrence risk and long-term prognosis. Am J Obstet Gynecol 1991;165:1408-1412.
- Meis PJ, Goldenberg RL, Mercer BM, et al. The preterm prediction study: Risk factors for indicated preterm births. Maternal-fetal medicine units network of the national institute of child health and human development. Am J Obstet Gynecol 1998;178:562-567.
- Shenhav S, Gemer O, Sassoon E, et al. Mid-trimester triple test levels in early and late onset severe pre-eclampsia. Prenat Diagn 2002;22:579-582.
- Onah HE, Iloabachie GC. Conservative management of early-onset pre-eclampsia and fetomaternal outcome in nigerians. J Obstet Gynaecol 2002;22:357-362.
- Banhidy F, Dakhlaoui A, Dudas I, et al. Birth outcomes of newborns after folic acid supplementation in pregnant women with early and late pre-eclampsia: A population-based study. Adv Prev Med 2011;127-369.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: Risk factors and outcomes associated with early versus late-onset disease. Am J Obstet Gynecol 2013;209:544.el-544.el2.
- Ebeigbe PN, Aziken ME. Early onset pregnancy-induced hypertension/eclampsia in benin city, Nigeria. Niger J Clin Pract 2010;13:388-393.
- Kenneth L, Hall DR, Gebhardt S, et al. Late onset preeclampsia is not an innocuous condition. Hypertens Pregnancy 2010;29:262-270.
- Akolekar R, Syngelaki A, Sarquis R, et al. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11-13 weeks. Prenat Diagn 2011;31:66-74.
- Eskenazi B, Fenster L, Sidney S, et al. A multivariate analysis of risk factors for preeclampsia. JAMA 1991;266:237-241.
- Coonrod DV, Hickok DE, Zhu K, et al. Risk factors for preeclampsia in twin pregnancies: A population-based cohort study. Obstet Gynecol 1995;85:645-650.
- Crispi F, Llurba E, Dominguez C, et al. Predictive value of angiogenic factors and uterine artery doppler for early versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol 2008;31:303-309.
- Murphy DJ, Stirrat GM. Mortality and morbidity associated with early-onset preeclampsia. Hypertens Pregnancy 2000;19:221-231.
- Sezik M, Ozkaya O, Sezik HT, et al. Expectant management of severe preeclampsia presenting before 25 weeks of gestation. Med Sci Monit 2007;13:523-527.
- Gasem T, Al Jama FE, Burshaid S, et al. Maternal and fetal outcome of pregnancy complicated by HELLP syndrome. J Matern Fetal Neonatal Med 2009;22:1140-1143.
- Makinde ON, Adegoke OA, Adediran IA, et al. Hellp syndrome: The experience at ile-ife, Nigeria. J Obstet Gynaecol 2009;29:195-199.
- Jenkins SM, Head BB, Hauth JC, et al. Severe preeclampsia at <25 weeks of gestation: Maternal and neonatal outcomes. Am J Obstet Gynecol 2002;186:790-795.
- Yeo S. A risk reduction model for late-onset preeclampsia: A theory for using low-intensity exercises to enhance cardiac homeostasis in nursing research and practice. Ans Adv Nurs Sci 2011;34:78-88.
- Bombrys AE, Barton JR, Habli M, et al. Expectant management of severe preeclampsia at 27(0/7) to 33(6/7) weeks gestation: Maternal and perinatal outcomes according to gestational age by weeks at onset of expectant management. Am J Perinatol 2009;26:441-446.