- Biomedical Research (2012) Volume 23, Issue 3
Markers of blood coagulation, lipid profile, renal function test and serum electrolytes in streptozotocin-induced diabetic rats.
Haseeb Ahmad Khan1*, Mohammad Shamsul Ola21Department of Biochemistry, College of Science, King Saud University, Riyadh, Saudi Arabia
2Department of Ophthalmology, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- *Corresponding Author:
- Haseeb Ahmad Khan
Department of Biochemistry
College of Science, Bld 5
King Saud University
P.O. Box 2455, Riyadh 11451
Saudi Arabia
Accepted May 30, 2012
Abstract
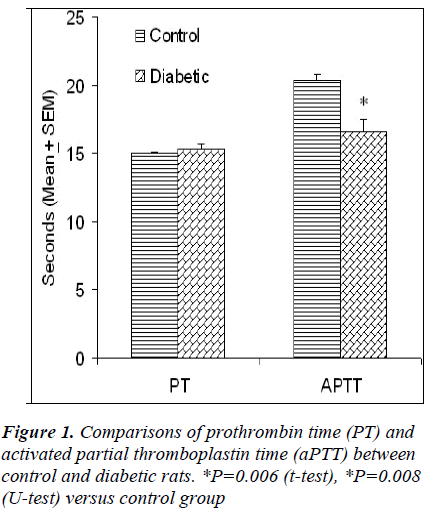
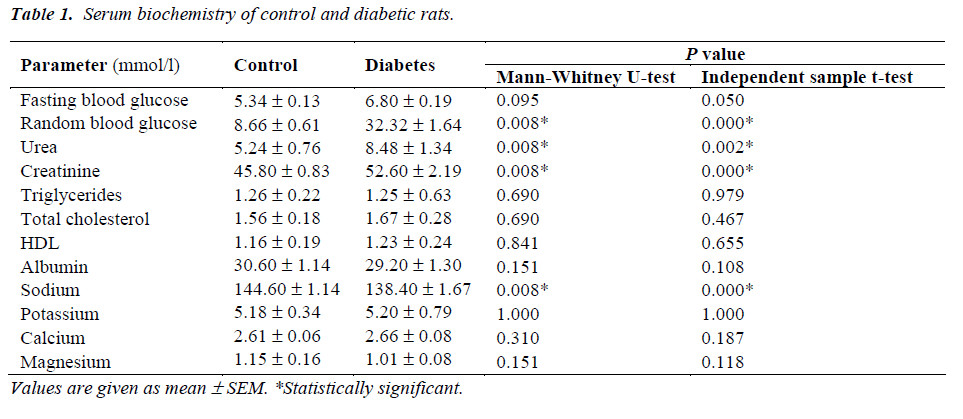
The presence of dyslipidemia, atherogenesis and thrombophilia in diabetic patients is well known. However, the presentation of these features in streptozotocin (STZ)-induced diabetic rats is a controversial issue; particularly the effect of STZ on blood coagulation tendency is not clearly known. This study reports the effect of STZ (45 mg/kg, ip) on blood glucose, renal function, lipids profile, serum electrolytes, albumin and blood clotting tendency in rats. Adult male Sprague Dawley rats were divided into two groups of five animals each. The random blood glucose (32.32 ± 1.64 versus 6.80 ± 0.19 mmol/l) was significantly higher in the diabetic rats as compared to control animals. There was no significant difference in prothrombin time (PT) of control (15.02 ± 0.07 s) and diabetic (15.34 ± 0.34 s) rats whereas activated partial thromboplastin time (aPTT) was significantly lower in diabetic rats (16.62 ± 0.94 s) than control animals (20.40 ± 0.40 s). Administration of STZ significantly increased serum urea and creatinine levels indicating impaired renal function in diabetic rats. There was no significant difference in serum lipids, albumin and electrolytes between the two groups except that a significant decrease in serum sodium was observed in diabetic rats. In conclusion, the findings of this study show that even a short-term (1 week) hyperglycemia induced by STZ significantly impairs the renal function and blood clotting tendency in rats. This animal model may have potential relevance for anticoagulation therapy testing.
Keywords
Experimental diabetes; Streptozotocin; Biomarkers; Renal function; Lipids profile; Blood coagulation; Electrolytes
Introduction
Besides hyperglycemia, diabetic patients also suffer from dyslipidemia [1], which can lead to increased atherogenesis and heart disease [2]. Clinical and epidemiological observations have led to the concept of a procoagulant state in type 2 diabetes. Thrombophilia in diabetic patients is a well recognized phenomenon which contributes an additional risk of coronary heart disease (CHD). In a series of 1980 type 2 diabetic patients, both male and female diabetic patients showed significantly shorter activated partial thromboplastin time (aPTT) [3]. Acang and Jalil [4] have reported significantly high fibrinogen and short prothrombin time (PT) and aPTT in diabetic patients, especially those who suffered from diabetes for a long time. Yurekli et al [5] have concluded a subtle activation of extrinsic pathway with a concomitant decrement in intrinsic pathway of the coagulation cascade in type 2 diabetics. Bae et al [6] observed that PT and aPTT did not differ significantly between diabetic patients and controls however a significant decrease was observed in fibrinogen levels in diabetic patients. Short aPTT and to a lesser extant short PT have been associated with increased risk for thromboembolism in renal transplant patients [7]. In a series of 35 type 1 diabetic patients, there was no significant difference in coagulation parameters and none of the patients developed thrombosis or vascular disease in a two year follow up [8].
Administration of streptozotocin (STZ) causes pancreatic beta cell destruction that leads to the development of hyperglycemia, dyslipidemia and renal dysfunction in rats [9]. The STZ animal model develops characteristic symptoms of diabetes such as hyperglycemia, hyperlipidemia and increased water and food intake without bodyweight gain. In addition, STZ diabetes also induces key symptoms including increased glycogen storage in the myocardium, depressed ventricular performance and cardiac hypertrophy [10]. Renal hypertrophy can be detected as early as one day after the onset of diabetes and seen regularly post 60-day of STZ injection [11]. It has been reported that diabetes-induced renal hypertrophy produces increased dimensions of renal cells along with increased kidney weight [12]. STZ diabetes also causes significant decrease in glycogen in liver and skeletal muscle as well as significant increase in glucose-6-phosphatase in hepatic tissue of rats [13]. This study reports the effect of STZ on serum biochemical parameters related to renal function, lipid profile, electrolytes and blood coagulation tendency.
Materials and Methods
Male Sprague-Dawley rats, weighing (250–300 g) were housed in a temperature-controlled room (24°C) with a 12-h light/dark cycle. Standard laboratory food and water were available ad libitum throughout the study. The rats were randomly divided into two groups of five animals each. One group of rats was rendered diabetic with a single i.p. injection of STZ (45 mg/kg) dissolved in normal saline, whereas the other group served as control and received saline only. After 7 days, blood glucose level was determined in fasted (15 h) and fed rats on consecutive days. For blood glucose analysis, the blood was taken via a tail nick, and the glucose concentration was determined with a Glucometer (Accutrend, Germany). The rats were then sacrificed and the blood was collected from heart for biochemical analysis. Serum cholesterol, lipoproteins, triglycerides, urea, creatinine, albumin and electrolytes were analyzed using an Autoanalyzer (Roche, Germany). The blood coagulation markers, PT and aPTT were measured by DiaPlastin and DiaClin kits (DiaMed GmbH, Switzerland).
The data were analyzed by both parametric (independent samples t-test) and nonparametric (Mann-Whitney U-test) tests using the SPSS version 10 software package. The P values < 0.05 were considered as statistically significant.
Results
The random blood glucose was significantly higher (32.32 ± 1.64 versus 6.80 ± 0.19 mmol/l) in diabetic rats as compared to control animals (Table 1). The fasting blood glucose in diabetic rats (8.66 ± 0.61) was not statistically significant as compared to control rats (5.34 ± 0.13 mmol/l). There was no significant difference in PT levels of control (15.02 ± 0.07 s) and diabetic (15.34 ± 0.34 s) rats whereas aPTT levels were significantly lower in diabetic rats (16.62 ± 0.94 s) than control animals (20.40 ± 0.40 s) (Fig. 1). Both urea and creatinine were significant- ly higher in the sera of diabetic rats as compared to control group (Table 1). There was no significant difference in serum lipids and electrolytes between the two groups except that a significant decrease in serum sodium was observed in diabetic rats as compared to control animals (Table 1).
Discussion
A single i.p. injection of STZ (45 mg/kg) in fasted rats produced hyperglycemia all the rats without causing any mortality (Table 1). Although the PT levels were unaffected by STZ treatment, a significant decrease in aPTT levels was observed in diabetic rats as compared to normal controls (Fig. 1). PT and aPTT respectively measures the extrinsic and intrinsic pathways of coagulation and are used to determine the bleeding or clotting tendency of blood. Significant increases in the markers of endothelial function including plasma soluble thrombomodulin (sTM), von Willebrand factor (vWF) and fibrinogen have been noticed in STZ-induced diabetes in rats [14]. Endothelial dysfunction and elevation of endothelial cell markers such as sTM may play a role in the initial stage of atherosclerosis [15]. In STZ diabetes, increase in plasma fibrinogen and decrease in aXIII factor have also been reported [16]. Thus, a shorter aPTT in STZ-treated rats suggest the possible use of this model for testing the effect of anticoagulant therapy.
Administration of STZ caused significant increases in serum urea and creatinine levels indicating massive renal dysfunction in diabetic rats (Table 1). Administration of STZ caused 2.66-fold and 1.87-fold increase in serum creatinine at 14-day and 28-day respectively [17]. Chen et al [17] observed that both 14-day and 28-day diabetic rats exhibited reduced renal blood flow along with 3-2 fold increase in plasma creatinine levels. Kang et al [18] observed a significant increase in serum urea without any significant change in serum creatinine in STZ-induced diabetic rats. This variation may be attributed to the dose of STZ, route of drug administration, age, sex and strain of rats and the duration of diabetes. Umerani and Goyal [19] demonstrated an increase in serum creatinine as an indicator of deteriorated renal function in diabetic rats. Itoh and coworkers [20] also demonstrated that serum creatinine levels in control group and diabetic group varied from 0.8 to 1.4 mg/dl respectively. Nogueira et al [21] observed significant increases in serum urea and creatinine and decrease in albumin in STZ (45 mg/kg) diabetes rats. Yokozawa et al [22] have reported significant increases in serum urea, creatinine and creatinine clearance in 50 days diabetic rats. Thus the results of our study are in accordance with earlier findings indicating diabetes-induced renal dysfunction in rats.
We did not observe any significant difference in the lipids profile and electrolytes (exceptsodium) between control and diabetic rats (Table 1). Earlier studies have shown a wide variation in the lipid profiles of STZ-induced diabetic rats; this may be attributed to the differences in age, weight, gender and species of rats as well as the dose and route of STZ exposure. Serum total cholesterol, TG and LDL were significantly increased and HDL significantly reduced at 14 day after STZ injection [13]. Haidara et al [14] observed significant reduction in HDL after 8 weeks of single intraperitoneal injection of STZ (65 mg/kg). Miguez et al [23] have reported significant increase in LDL and TG at 4 weeks in STZ diabetic rats. Anandh Babu et al [24] have found significant increase in serum levels of cholesterol, TG and LDL and decrease in HDL at 30 days after single ip injection of STZ (60 mg/kg). Tuanli and Yanardag [25] have reported similar observations at 60 days after single ip injection of STZ (65 mg/kg). Holmgren and Brown [26] failed to observe any significant difference in plasma cholesterol between control and STZ diabetic rats, whereas STZ diabetic rats fed on cholesterol rich diet exhibited significant hypercholesterolemia as compared to normal rats fed on the same diet. Zhu et al [27] did not find any significant difference in serum LDL, HDL and TG at 6 and 12 weeks following STZ treatment.
In conclusion, a single injection of STZ induces a permanent hyperglycemia in rats. These rats also show significant impairment in the renal function and blood clotting tendency. However, the levels of serum lipids, albumin and electrolytes (except sodium) were similar between the control and diabetic groups. The findings of this study point towards the possible utilization of this animal model for anticoagulation therapy testing however further studies are needed for its standardization and verification.
Acknowledgments
The author extends his appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGPVPP- 052.
References
- Khan HA, Sobki SH, Khan SA. Association between glycaemic control and serum lipids profile in type 2 dia- betic patients: HbA1c predicts dyslipidaemia. Clin Exp Med 2007; 7: 24-29.
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabe- tes, other risk factors and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Interven- tion Trial. Diab Care 1993; 16: 434-444.
- Chan P, Pan WH. Coagulation activation in type 2 dia- betes mellitus: the higher coronary risk of female dia- betic patients. Diabet Med 1995; 12: 504-507.
- Acang N, Jalil FD. Hypercoagulation in diabetes melli- tus. Southeast Asian J Trop Med Public Health 1993; 24: 263-266.
- Yurekli BP, Ozcebe OI, Kirazli S, Gurlek A. Global assessment of the coagulation status in type 2 diabetes mellitus using rotation thromboelastography. Blood Coagul Fibrinolysis 2006; 17: 545-549.
- Bae SH, Lee J, Roh KH, Kim J. Platelet activation in patients with diabetic retinopathy. Korean J Ophthal- mol 2003; 17: 140-144.
- Guirguis NG, Eicher C, Hock L, Lynch J, Graham VD, Rajagopalan PR, et al. Thromboembolic risk factors in patients undergoing kidney transplant: implication of abnormally short activated partial thromboplastin time. Ann Clin Lab Sci 2003; 33: 396-400.
- Zeitler P, Thiede A, Muller HL. Prospective study on plasma clotting parameters in diabetic children - no evidence for specific changes in coagulation system. Exp Clin Endocrinol Diab 2001; 109: 146-150.
- Clozel M, Hess P, Qiu C, Ding SS, Rey M. The uro- tensin-II receptor antagonist palosuran improves pan- creatic and renal function in diabetic rats. J Pharmacol Exp Ther 2006; 316: 1115-1121.
- Mihm MJ, Seifert JL, Coyle CM, Bauer JA. Diabetes related cardiomyopathy time dependent echocardio-graphic evaluation in an experimental rat model. Life Sci 2001; 69: 527-542.
- Seyer Hansen K. Renal hypertrophy in streptozotocin diabetic rats. Clin Sci Mol Med Suppl 1976; 51: 551- 555.
- Tang D, Yu T, Khraibi AA. Cardiovascular and renal characteristics and responses to acute volume expan- sion of a rat model of diabetic pregnancy. Life Sci 2004; 74: 2909-2918.
- Maiti R, Das UK, Ghosh D. Attenuation of hypergly- cemia and hyperlipidemia in streptozotocin-induced di- abetic rats by aqueous extract of seed of Tamarindus indica. Biol Pharm Bull 2005; 28: 1172-1176.
- Haidara M, Khlonssy H, Ammar H, Kassem LA. Imp- act of a-tocopherol and vitamin C on endothelial mark- ers in rats with streptozotocin-induced diabetes. Med Sci Monit 2004; 10: 41-46.
- Aso Y, Fujiwara Y, Tayama K, Takanashi K, Inukai T, Takemura Y. Relationship between plasma soluble thrombomodulin levels and insulin resistance syndrome in type 2 diabetes: comparison with von Willebrand factor. Exp Clin Endocrinol Diab 2001; 109: 210-216.
- Nishigaki A. Experimental studies of skin wound heal- ing process by first intention in streptozotocin-induced diabetes mellitus rats. Shikwa Gakuho 1989;89:793- 822.
- Chen H, Brahmbhatt S, Gupta A, Sharma AC. Duration of streptozotocin-induced diabetes differentially affects p38-mitogen-activated protein kinase (MAPK) phos- phorylation in renal and vascular dysfunction. Cardio- vasc Diabetol 2005; 4: 3-15.
- Kang KS, Kim HY, Yamabe N, Nagai R, Yokozawa T. Protective effect of Sun ginseng against diabetic renal damage. Biol Pharm Bull 2006; 29:1 678-1684.
- Umrani DN, Goyal RK. Fenoldopam treatment impro- ves peripheral insulin sensitivity and renal function in STZ-induced type 2 diabetic rats. Clin Exp Hyperten-sion 2003; 25: 221-233.
- Itoh Y, Imamura S, Yamamoto K, Ono Y, Nagata M, Kobayashi T, et al. Changes of endothelin in strepto- zotocin-induced diabetic rats: effects of an angiotensin converting enzyme inhibitor, enalapril maleate. J Endo- crinol 2002; 175: 233-239.
- Nogueira Junior FC, Coelho DA, Almeida MM, Silva TC, Ferreira EC, Macedo UB, et al. Effect of tamoxifen in lipids of diabetic rats induced by streptozotocin. Ac- ta Cir Bras 2005; 20: 114-120.
- Yokozawa T, Nakagawa T, Oya T, Okubo T, Juneja LR. Green tea polyphenols and dietary fibre protect against kidney damage in rats with diabetic nephropa- thy. J Pharm Pharmacol 2005; 57: 773-780.
- Miguez I, Marino G, Rodriguez B, Taboada C. Effects of dietary L-arginine supplementation on serum lipids and intestinal enzyme activities in diabetic rats. J Phy- siol Biochem 2004; 60: 31-37.
- Anandh Babu PV, Sabitha KE, Shyamaladevi CS. Green tea extract impedes dyslipidaemia and develop- ment of cardiac dysfunction in streptozotocin-diabetic rats. Clin Exp Pharmacol Physiol 2006; 33: 1184-1189.
- Tunali S, Yanardag R. Effect of vanadyl sulfate on the status of lipid parameters and on stomach and spleen tissues of streptozotocin-induced diabetic rats. Pharma- col Res 2006; 53: 271-277.
- Holmgren PR, Brown AC. Serum cholesterol levels of nondiabetic and streptozotocin-diabetic rats fed a high cholesterol diet. Artery 1993; 20: 337-245.
- Zhu B, Shen H, Zhou J, Lin F, Hu Y. Effects of simvas- tatin on oxidative stress in streptozotocin-induced dia- betic rats: a role for glomeruli protection. Nephron Exp Nephrol 2005; 101: e1-8.