Mini Review - International Journal of Pure and Applied Zoology (2021) Volume 9, Issue 5
MARINE GREGARINES (APICOMPLEXA): THEIR BIOLOGY, IDENTIFICATION AND CONTROL
Mohd Ihwan Zakariah1*, Hassan Mohd Daud2, Mhd. Ikhwanuddin1,3, Nor Asma Husna Yusoff1, Marina Hassan1,3
1Department of Veterinary Medicine, Higher Institution Centre of Excellence (HICoE), Institute of Tropical Aquaculture and Fisheries (AKUATROP), Universiti Malaysia Terengganu (UMT), 21030 Kuala Nerus, Terengganu, Malaysia
2Department of Veterinary Medicine, Universiti Putra Malaysia (UPM), 43400 UPM Serdang, Selangor, Malaysia
3Department of Veterinary Medicine, STU-UMT Joint Shellfish Research Laboratory, Shantou University, Shantou, 515063, China
- Corresponding Author:
- Mohd Ihwan Zakariah
Department of Veterinary Medicine, Higher Institution Centre of Excellence (HICoE), Institute of Tropical Aquaculture and Fisheries (AKUATROP), Universiti Malaysia Terengganu (UMT)
21030 Kuala Nerus, Terengganu
Malaysia
E-mail: ihwanz@umt.edu.my
Received 09th September, 2021; Accepted 23rd September, 2021; Published 30th September, 2021
Citation: Farvardin M, Johari MK, Nami M, et al. Annular choroidal detachment one year after argus-II retinal prosthes is implantation. Ophthalmol Case Rep. 2020;4(1):23-25.
Abstract
Gregarine (Apicomplexa) is not a new-fangled topic of study that needs to be discussed nowadays. This research has been explored since the 1700s by European expertise. Gregarines are obligate unicellular parasites that infect the intestines, reproductive organs and body cavities of invertebrates living in terrestrial, freshwater and marine habitats. These parasites form resistant cysts that appear to be ubiquitously distributed throughout marine, freshwater and terrestrial sediments. The systematics of the group is organized into three traditional categories based more on convenience than phylogenetic relationships: eugregarines, archigregarines, and neogregarines. But lately, this research is become important due to the problem reported by this parasite especially in commercial bivalve i.e., oyster. A preliminary study on gregarine parasite showed a high prevalence of infection in Hairy Cockle (Anadara cornea) from Setiu Lagoon, East Coast of Peninsular Malaysia. Due to the advanced technologies nowadays, this research regarding these parasites has become relevant to study. This review described the morphology, life cycle, method for identification, parasites transmission and ways for control and prevent this gregarine parasites from spread into aquaculture. Other previous research on other gregarine parasitized hosts also were summarized. Details and diagnoses of these parasites in aquatic animals are crucial to secure the sustainability of aquaculture industries in the future.Keywords
Gregarines, Apicomplexa, Protozoan, Aquatic animals, Aquaculture
Introduction
Nowadays, the seafood consumption demand by a human is increase throughout the year. However, the commonly cultured species did not support the demand and now a lot of new species are introduced for species culture including crustaceans. Farmed and captured crustaceans (shrimp, crabs, lobsters, etc.) contribute a significant proportion of global seafood production. The aquaculture industries support rural livelihoods and poverty alleviation in producing nations within Asia and Latin America and contribute to the aquatic food supply in developed nations. Nations with marine borders commonly support important marine fisheries for crustaceans that are regionally traded as live animals and commodity products.
At the first stage of introducing new species to aquaculture, the common step is the collection of species broodstock/ berried female from wild. This is the critical step where the disease/parasite-attached to the animals would be brought together, and increase the tendency to be separated and grow in the aquaculture industry. This issue has been identified as a significant bottleneck for the successful harvesting of wild and cultured crustaceans nowadays.
The cephalin gregarines generally are not noted for being highly detrimental to their hosts, the best-known effect being damage done to the epithelial cells to which the young trophozoites are attached in the intestine of the host (Walker et al., 2007). This effect, whatever it may be, is directly related to the number of parasites present since each parasite attacks a single host cell. The number present at a given time is limited by two important factors inherent in the process of reproduction. In some species, the potential effect of the parasite on the intermediate (molluscan) host is of particular interest because of their economic importance as for example is oyster. This parasite also probably has definitive host (i.e. crustaceans) to complete their life cycles (Rueckert et al., 2011); (Criado-Fornelio et al., 2017). Here, again, there is no known multiplication within the host but each parasite (spore) infecting the oyster is acquired as an original invader. Any effect of the parasite on the host must, therefore, be caused by these original invaders; there can be no effect (as in most Sporozoa and pathogenic bacteria) attributable to a multiplication of the parasite within the oyster.
Spores of gregarine, unlike the vegetative stages, may cause the possibility of a cumulative effect on the host. It also seems quite possible that such an effect may accompany the progressive mechanical obstruction of the blood vessels of the organs involved. It is also conceivable, as the suggestion by Valigurova (Valigurova et al., 2012) that toxic end products of metabolism might be liberated into the host tissues by the developing sporoblasts. Therefore, this review reflects the current state-of-art on the field of crustacean diseases, particularly on gregarine (Apicomplexa), their biology, a recent method for identification and ways for control and prevention.
Morphology of Gregarines
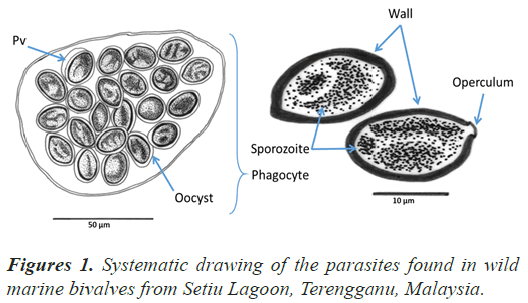
Comparative gregarine cell morphology placed in a modern phylogenetic context helps clarify the earliest stages of apicomplexan evolution and demonstrates novel ways (Perkins et al., 2000); (Wei et al., 2014), in which these parasites have solved fundamental biological problems including locomotion (nematode-like bending movements, gliding motility and peristalsis), host-dependent nutrition (myzocytosis, nutrition absorption via the surface) and reproduction (Walker et al., 2007). In the present study, the gregarine has been found infected the gill and intestine tissue of the Anadara cornea. The illustrated of the parasites are shown in Figure 1. From the analysis, the infection intensity was high and each phagocyte (Pha) contain a maximum of 15 oocysts (Oc). Each oocyst has a single cell wall, longitudinal shape and contained sporozoite (Sz). Parasitophorous vacuole (Pv) covered by a membrane wall.
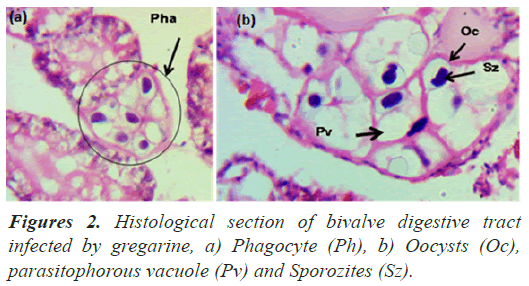
The biodiversity and phylogenetic relationships of gregarines are very poorly understood and only a small minority of the (predicted) total number of extant gregarine species have been described so far. Meanwhile, a less than 1% of known invertebrates have been examined for gregarine infection (Golemansky et al., 2015), (Uddin et al., 2010). A compounding issue is that only a tiny number of described gregarine species have been studied at the molecular level, which makes species delimitation (using molecular barcodes) and inferences about broader phylogenetic relationships among gregarines become very difficult (Leander et al., 2007). Figures 2a and 2b show a bivalve digestive tract after infected by gregarine viewed under a microscope with magnification of 400X. Oocyst is commonly found in the parasitized phagocytes. Further analysis will be correlated with the tissue changes by using lesion scoring obtained from the former study. A previous study of oocyst also reported by (Clopton et al., 2016).
Lifecycle of Gregarines
Gregarine is ubiquitous and mostly found infected in both aquatic and terrestrial environment. Hence, these parasites are transmitted commonly by the faecal-oral route path and also by copulation. Moreover, some oocysts are developed in sporozoites which the process called excystation. This stage was the best-developed stages of these parasites for them to find a suitable host (Rueckert et al., 2008). Most of the gregarine lifecycle are comprise the host in the terrestrial and aquatic environment to complete their lifecycle. In this present study, gregarine parasites that have been studied are focused on aquatic species parallel to the importance of this parasites to aquaculture.
Reproduction of Gregarines
Eugregarine (which category Nematopsis spp. belongs to) are peculiar in having no asexual reproduction (Simdyanov et al., 2017). The vast majority of the parasitic protozoa (as well as pathogenic bacteria) undergo some type (or types) of asexual reproduction (binary fission, multiple fission, budding, plasmotomy, etc.), to produce large numbers of new individuals like the parents who are capable of spreading the infection within the body of the host. These unique protozoa so-called Eugregarinina, reproduce only by sporogony (sexual reproduction) and the products leave the body of the host to perish or enter again into the same or another host (Criado-Fornelio et al., 2017). Thus, these parasites do not increase numerically within the host by reproduction. In this respect, they are comparable to many helminth parasites rather than protozoa. Unlike the helminthes, however, the adult gregarines themselves perish in the process of sexual reproduction; this is the second limiting factor, mentioned above, in the numbers of parasites in the host (Rueckert et al., 2011). Therefore, the reproductive process in these gregarines not only fails to increase but decreases the numbers of individuals present in the host (Leander et al., 2007). This process causes complete and rapid (the time being a function of the temperature) elimination of the gregarines present at a given time and the infection, if it is maintained, must be replenished by new invaders. The number of parasites (and possibly their effect), therefore, fluctuate and the severity of their effect may depend very largely on whether there is repeated and frequent acquisition of new parasites. Acquisition of great numbers possibly results in damage to the host other than the destruction of epithelial cells as mentioned above. The sheer bulk of numbers may probably damage the intestine mechanically or interfere with the proper function. The above remarks apply either to certain gregarines which have only one host or to those stages of the Porosporidae occurring in the definitive host (the one in which sexual reproduction occurs, the decapod host of Porosporidae).
Gregarine Movement and Infection
The effect of gregarine infections in aquatic organisms nowadays is reported due to the importance of these parasites. This information is important due to the effect of this parasites which they can give a serious impact on the sustainability of aquaculture in the future. For the movement to fulfil the life cycle of this parasites, some study explained the gregarine can move and can change the direction along the surface through gliding movement (Lavilla-Pitogo et al., 2001); (Wakeman et al., 2014) without the use of any cilia and flagella. Previous researcher proof that the motility is happen using actin and myosin complex in the complexity of actin cytoskeletons to perform the gliding motion (David et al., 2015). Some study stated that the epicytes of all the gregarines are differentiated into a system of regular longitudinal folds. Some septate species fold undulate so that these parasites can glide along.
Recent Methodology for Identifications
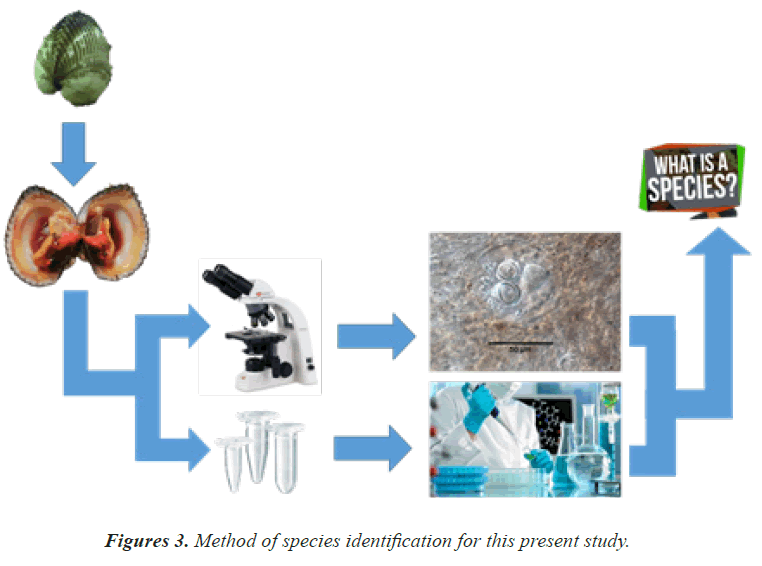
Marine gregarines (Apicomplexa) are a diverse but poorly understood assemblage of endoparasites that infect the intestines and other extracellular spaces in a wide range of marine invertebrates (Rueckert et al., 2011); (Simdyanov et al., 2017); (Lavilla-Pitogo et al., 2001). Table 1 summarized some of the previous aquatic animals infected by gregarines (Prasadan et al., 2001); (Silva et al., 2019); (Erazo-Pagador et al., 1932). Only a tiny fraction of the known diversity of marine gregarines is represented in molecular phylogenetic data sets; the most widely explored marker so far has been small-subunit (SSU) rDNA sequences (Leander et al., 2007), (David et al., 2015), (Erazo-Pagador et al., 2010), (Leander et al., 2006). Nonetheless, phylogenetic analyses of DNA sequences used in tandem with high-resolution microscopy of trophozoite stages has helped shape our understanding of gregarine diversity and evolutionary history. This approach has also been vital for the delimitation and identification of different gregarine species and for establishing the cellular identities of ambiguous environmental DNA sequences generated from several different PCR surveys of marine biodiversity (Kuan and Taha) (Leander et al., 2006). The previous study also reported more on morphological characteristic using Transmission Electron Microscopes (TEM) (Leander et al., 2008). Figure 3 showed the method of species identification which has been used in this study. (A) Sample was collected from the wild and the measurement for weight, length and width were recorded. (B) Sample were dissected with standard diagnosis procedure. (C) A piece of tissue from the sample were cut and placed on the slide and observed under light microscope. (D) Ten millimetre of tissue sample were cut and fix in 100% ethanol for genetic study. (E) The target parasite was counted and captured for further identification purposes. (F) The fix tissue was undergoing standard genetic analysis study. (G) Result from parasitology and genetic analysis were combined for details of morphological identification of the species. The morphological identification was referred to the previous published data from expert. The standard primer for DNA identification in this study is Universal Eukaryote Primer (UniEP). For the prevalence and mean intensity of the parasites, the standard method of analysis refers to previous research published worldwide (Ihwan et al., 2013) (Ihwan et al., 2017).
| Species | Host species | Group of host | Origin | References |
|---|---|---|---|---|
| Ganymedes yurii | Gondogeneia sp. | Amphipod | James Ross Island, Weddell Sea, Antarctica | (Diakin et al., 2016) |
| Polyplicarium lacrimae | Notomastus tenuis | Polychaete | Boundary Bay, Tsawwassen | (Wakeman et al., 2913) |
| Polyplicarium curvarae | ||||
| Polyplicarium translucidae | ||||
| Polyplicarium citrusae | Heteromastus filiformis | Jericho Beach, Vancouver | ||
| Nematopsis | Parapeneopsis stylifera | Shrimp | Khouzestan Province, Iran | (Zanguee et al., 2011) |
| Rotundula | ||||
| Heliospora | ||||
| Cephaloidophora conformis | Pachygrapsus marmoratus | Crab | The coast of Portugal | (Kuris et al., 2002) |
| Cephaloidophora fossor | Pinnotheres pisum | Pea crab | English Coastline | (Longshaw et al., 2011) |
| Nematopsis sp. | Penaeus monodon | Black tiger shrimp | Commercial farms in Thailand | (Poulpanich et al., 2009) |
| Thiriotia pugettiae | Pugettia gracilis | Kelp crab | Vancouver Island, Canada | (Rueckert et al., 2011) |
| Cephaloidophora communis | Balanus glandula | Barnacles | ||
| Balanus balanus | Moscow | |||
| Heliospora longissima | Eulimnogammarus verrucosus | Freshwater amphipods | Lake Baikal | |
| Eulimnogammarus vittatus | ||||
| Heliospora caprellae | Caprella alaskana | Skeleton shrimp | Vancouver Island | |
| Nemaptosis marinum | Litopanaeus vannamei | Shrimp | Ecuador | (Jimenez et al., 2002) |
| Nematopsis mytella | Crassostrea rhizophorae | Oyster | Brazil | (Padovan et al., 2003) |
| Nematopsis sp. | Callista chione | Clam | Italy | (Canestri-Trotti et al., 2000) |
| Anadara granosa | Blood cockle | Thailand | (Tuntiwaranuruk et al., 2004) | |
| Malaysia | (Uddin et al., 2010) | |||
| Perna veridis | Green muscle | Thailand | (Tuntiwaranuruk et al., 2004) | |
| Perna veridis | Malaysia | (Kua et al., 2004) | ||
| Crassostrea rhizophorae | Oyster | Brazil | (Silva et al., 2019) | |
| Arcuatula arcuatula | Clam | Thailand | (Tuntiwaranuruk et al., 2004) | |
| Paphia undulata | Mussel | Thailand | ||
| Crassostrea irredilai | Tropical oyster | Philippines | (Erazo-pagador et al., 2010) | |
| Malaysia | (Kua et al., 2004) | |||
| Nematopsis messor | Metapograpsus messor | Estuarine crab | Kerala, India | (Prasadan et al., 2001) |
| Nematopsis quadratum | Sesarma quadratum | |||
| Nematopsis annulipes | Uca annulipes |
Table 1: Previous reports on gregarine infection in aquatic species.
Control and Prevention
Recommendations for the preventions of the infections of gregarines seem to be done as host are retaining their infections for considerably longer periods if they were put together than do similar isolated ones. No infected organisms have been found among those forms commonly associated with the crab, such as barnacles or mussels, which might be suspected as serving as possible intermediate hosts. Infection by the discharge of resistant stages into the seawater is uncommon if it occurs at all normally since the parasite is rarely found in the posterior part of the hindgut or the faeces of the host. Furthermore, the effect of gregarine on the aquatic animal seems to be limited to the destruction of the epithelial and cuticle lining of the host’s gut by the mechanical action of the parasites. The epithelial lining becomes thinned out beneath the point of attachment of the parasite, or in other cases, it may cause sloughed off, especially if heavy infections. Moreover, gregarine does not appear to penetrate the cells, it must obtain its food supply from the lumen of the digestive tract. Hence, an exceedingly heavy infection might destroy sufficient tissue to inconvenience the host. It is doubtful if this would occur normally even in severe infections, comparatively few of the host's epithelial cells seem to have been materially injured.
For Nematopsis sp., the source of the shrimp seed may be an important factor because wild post larvae had higher survival and lower prevalence of infection then laboratory- reared post larvae. Medicated feeds that were tested did not alleviate the infection or improve survival. The source of Paraophioidina scolecoides was not identified but the shrimp acquired the infection at the culture facility. Because gregarines require at least two hosts (usually a mollusc or annelid worm in addition to the crustacean) to complete their life cycle, the infection can be circumvented by removal of the alternate host(s) from the culture facility or water source41. Study of proper prophylaxis and treatment are not reported yet especially for this parasite. The procedures of disease prevention already mentioned by (Wei et al., 2014); (Lavilla-Pitogo et al., 2001); (Ihwan et al., 2013); (Leander et al., 2003) (Noga et al., 2000). Hence, this review could be the best “booster” to solve the problem on the infection of these gregarine parasites.
Conclusion
Although gregarine is not reported as the main problem to aquaculture. But, most of the countries that have this problem previously had re-strategized their plan to overcome the high mortality rate caused by the infections of this gregarine in aquaculture industries. Many Standards of Procedures (SOP) have been developed by a certain country that is facing this problem including Malaysia. This is not an insignificant issue today, however will appear to cause a huge outbreak in aquaculture industries in the future if no further action is taken.
Significance Statement
Through this review, it can be beneficial for both researchers and people in the aquaculture industry to uncover the current identification method for gregarine detection in crustaceans which are scarce. Thus, a new policy-related issue associated with the trans-global movement of crustaceans and the potential disease mitigation strategies might be improved to prevent the significant losses inherent within the aquaculture industry caused by this gregarine parasite.
Acknowledgement
Abundant thanks to the Institute of Tropical Aquaculture (AKUATROP) and Universiti Malaysia Terengganu (UMT) for allowing me to continue my study. Also, to staff in management department, Faculty of Veterinary Medicine, Universiti Putra Malaysia (UPM) for their acceptance of my registration of study and willingness to help me to manage my study. Also to all staff in UPM and UMT, who are willing to support me physically and spiritually especially to Mrs Farizan Abdullah, Mrs Wahidah Wahab, Mr Ahmad Shuhaimi Draman, Mr Ahmad Najmi Ishak, Dr Mohd Fuad, Mrs Latifah and people who had help me directly or indirectly.
References
- Walker M.H., Mackenzie, C., Bainbridge, S.P., and Orme, C. (2007). A study of the structure and gliding movement of Gregarina garnhami, J Protozool., 24(4): 566-574.
- Rueckert, S., Simdyanov, T.G., Aleoshin, V.V., and Leander, B.S. (2011). Identification of a divergent environmental DNA sequence clade using the phylogeny of gregarine parasites (Apicomplexa) from crustacean hosts. PLoS ONE, 6(3): e18163.
- Criado-Fornelio, A., Verdu-Exposito, C., Martin-Perez, T., Heredero-Bermenjo, I., Perez-Serrano, J., Guardia-Valle, L., and Panisello-Panisello, M. (2017). A survey for gregarines (Protozoa: Apicomplexa) in arthropods in Spain. Parasitol Res., 116: 99-110.
- Valigurova, A., 2012. Sophisticated adaptations of Gregarina cuneata (Apicomplexa) feeding stages for epicellular parasitism. PLOS One., 7(8): e42606.
- Perkins, F.O., Barta, J.R., Clopton, R.E., Pierce, M.A. and Upton, S.J. (2000). Phylum Apicomplex a. – In: Lee J. J., G. F. Leedale, and P. Bradbury 2000. An Illustrated Guide to the Protozoa: organisms traditionally referred to as Protozoa, or newly discovered groups. I. (2nd ed.), Society of Protozoologists, Allen Press inc Lawrence., 190-200.
- Wei, L.S., and Wee, W. (2014). Diseases in aquaculture. Res Vet Sci., 7(1): 1-6.
- Golemansky, V. (2015). Checklist of Gregarines (Apicomplexa: Eugregarinorida and Neogregarinorida) from Bulgaria. Acta zool Bulg., 67(2): 149-157.
- Uddin, M. Jasim, Zulfigar, Y., Munawar, K., and Shau-Hwai, A.T. (2010). Parasites of blood cockle (Anadara granosa Linnaeus, 1758) from the Straits of Malacca. J Shellfish Res., 30(3): 875-880.
- Leander, B.S. (2007). Molecular phylogeny and ultrastructure of Selenidium serpulae (Apicomplexa, Archigregarinia) from the calcareous tubeworm Serpula vermicularis (Annelida, Polychaeta, Sabellida). Zool Scr., 36, 213–227.
- Clopton, R.E., Steele, S.M., and Clopton, D.T. (2016). Environmental persistence and infectivity of oocysts of two species of gregarines, Blabericola migrator and Blabericola cubensis (Apicomplexa: Eugregarinida: Blabericolidae), parasitizing Blaberid coackroaches (Dictyoptera: Blaberidae). J Parasitol., 102(2): 169-172.
- Rueckert, S., and Leander, B.S. (2008). Morphology and phylogenetic position of two novel marine gregarines (Apicomplexa, Eugregarinorida) from the intestines of North Eastern Pacific ascidians. Zool Scr., 37: 637-645.
- Simdyanov, T.G., Guillou, L., Diakin, A.Y., Mikhailov, K.V., Schrevel, J., and Aleoshin, V.V. (2017). A new view on the morphology and phylogeny of eugregarines suggested by the evidence from the gregarine Ancora sagittata (Leuckart, 1860) Labbe, 1899 (Apicomplexa: Eugregarinida). Peer J., 5: e3354.
- Lavilla-Pitogo, C.R., Marcia1, H.S., Pedrajas, S.A.G., Quinitio, E.T., and Millamena, O.M. (2001). Problems associated with tank-held mud crab (Scylla spp.). Asian Fish Sci., 14: 217-224.
- Wakeman, K.C., and Leander, B.S. (2013). Identity of environmental DNA sequences using descriptions of four novel gregarine parasites, Polyplicarium n. gen. (Apicomplexa), from capitellid polychaetes. Mar Biodiv., 43: 133–147.
- David, B., Stentiford, G.D., Littlewood, D.T.J., and Hartikainen, H. (2015). Diverse Applications of Environmental DNA Methods in Parasitology. Tre Parasitol. 31(10): 499-513.
- Perkins, F.O., Barta, J.R., Clopton, R.E., Pierce, M.A. and Upton, S.J. (2002). Phylum Apicomplexa. In: Lee, J.J., Leedale, G.F. and Bradbury, P. (Eds). The Illustrated Guide to the Protozoa. Lawrence, Allen Press, Inc., pp: 190-304.
- Prasadan, P.K., and Janardanan, P.K. (2001). Three new species of gregarines (Apicomplexa: Sporozoea: Porosporidae) in the estuarine crabs from Kerala, India. Acta Protozool., 40: 303-309.
- Diakin, A., Wakeman, K.C., and Valigurova, A. (2016). Description of Ganymedes yurii sp. n. (Ganymedidae), a new gregarine species from the Antarctic amphipod Gondogeneia sp. (Crustacea). J Eukaryot Microbiol., 64: 56-66.
- Zanguee, N., Mokhayer, B., Mousavi, H.A.E., and Yavari, V. (2011). Study of gregarine infection in Kiddi shrimp (Parapenaeopsis stylifera) and Pacific white shrimp (Litopenaeus vannamei) in Khouzestan province. J. Vet. Res., 66(1): 55-60.
- Kuris, A.M., Torchin,M.E., and Lafferty, K.D. (2004). Parasites in the thoracic ganglion of Pachygrapsus marmoratus (Brachyura: Grapsidae) from the coast of Portugal. Parasite., 11: 425-427.
- Longshaw, M., Feist, S.W., and Bateman, K.S., 2011. Parasites and pathogens of the endosymbiotic pea crab (Pinnotheres pisum) from blue mussels (Mytilus edulis) in England. J. Invertebr. Pathol., 109: 235-242.
- Poulpanich, N. and Withyachumnarnkul, B. (2009). Fine structure of a septate gregarine trophozoite in the black tiger shrimp Penaeus monodon. Dis Aquat Org., 86: 57-63.
- Jimenez, R., Barniol, L.D., and Machuca, M. (2002). Nemaptosis marinus n. sp. A new septate gregarine from cultured panaeid shrimp Litopanaeus vannamei (Boone), Ecuador. Aquac. Res., 33: 231-240.
- Padovan, I.P., Corral, L., Tavares, L.A., Padovan, P.A., and Azevedo, C. (2003). Fine structure of the oocyst of Nematopsis mytella (Apicomplexa, Porosporidae), a parasite of the mussel Mytella falcata and of the oyster Crassostrea rizophorae (Mollusca, Bivalvia) from the northeastern Atlantic coast of Brazil. Braz J Morphol Sci., 20: 121-124.
- Canestri-Trotti, G., Baccarani, E.M., Paesanti, F., and Turolla, E. (2000). Monitoring of infections by Protozoa of the genera Nematopsis, Perkinsus and Porospora in the smooth venus clam Callista chione from the North-Western Adriatic Sea (Italy). Dis Aquat Org., 42: 157-161.
- Tuntiwaranuruk, C., Chalermwat, K., Upatham, E.S., Kruatrachue, M., and Azevedo, C. (2004). Investigation of Nematopsis spp. oocysts in 7 species of bivalves from Chonburi Province, Gulf of Thailand. Dis Aquat Org., 58: 47-53.
- Kua, B.C., and Taha, M.S. (2004). A preliminary observation of parasitic infestation on blood cockles (Anadara granosa) and tropical oyster (Crassostrea iredalei) in northern peninsular Malaysia. Mal Fish J, 3: 125-129.
- Silva, T.J., Soares, E.C., Casal, G., Rocha, S., Santos, E.L., Nascimento, R., Oliveira, E., and Azevedo, C. (2019). Ultrastructure of phagocytes and oocycts of Nematopsis sp. (Apicomplexa, Porosporidae) infecting Crassostrea rhizophorae in Northeastern Brazil. Braz J Vet Parasitol., 28(1): 97-104.
- Erazo-Pagador, G. (2010). A parasitological survey of slipper-cupped oyster (Crassostrea iredalei Faustino, 1932) in the Philippines. J Shellfish Res., 29: 177-179.
- Leander, B.S., Lloyd, S.A.J., Marshall, W., and Landers, S.C. (2006). Phylogeny of marine gregarines (Apicomplexa) — Pterospora, Lithocystis and Lankesteria – and the origin(s) of coelomic parasitism. Protist, 157: 45-60.
- Leander, B.S. (2008). Marine gregarines-evolutionary prelude to the apicomplexan radiation. Trends Parasitol., 24: 60-67.
- Ihwan, M.Z., Shaharom-Harrisson, F., Marina, H., and Wahidah, W. (2013). A comparative prevalence study of ectoparasites in wild and cultured grouper before and after transportation. J Sustai Sci Manag., 8(1): 121-125.
- Ihwan, M.Z., Ikhwanuddin, M., Somrit, T., Wahidah, W., and Marina, H. (2014). Parasites and Ecto-Symbiont of Mud Spiny Lobster, Panulirus polyphagus from Peninsular Malaysia. 4th International Fisheries Symposium, Surabaya, Indonesia.
- Ihwan, M. Z., Ikhwanuddin, M., Ambak, M. A., Shuhaimi, A. D., Wahidah, W. and Marina, H. (2015a). Study on the attachment of Octolasmis spp. on gill of wild mud crabs, genus Scylla from Setiu Wetland, Terengganu, Malaysia. Poult Fish Wildl Sci., 3: 142.
- Ihwan, M.Z., Wahidah, W., Ambak, M.A., Ikhwanuddin, M., and Marina, H. (2015b). Investigation of parasites and ecto-symbiont in wild mud crab, genus Scylla from Terengganu coastal water, Malaysia: rrevalence and mean intensity. Int J Zool Res., 11(4): 151-159.
- Ihwan, M.Z., Ikhwanuddin, M. and Marina, H. (2016a). Epibiosis of Pedunculate Barnacle Octolasmis spp. of wild Scylla spp. Books. Lambert Academic Publishing. ISBN 978-3-659-97945-3.
- Ihwan, M.Z., Wahidah, W., Marina, H., Shaharom-Harisson, F., Rabi-Atun, A.A., and Suhairi, M. (2016b). A case study of fish distress: prevalence and mean intensity of parasites. Agric. J, 1(3): 56-60.
- Ihwan, M.Z., Ambak, M.A., Wahidah, W. Nurul-Ulfah, K., Fakhrulddin, I.M., Marina, H. (2016c). A new Temnocephalid trematode report from freshwater crustaceans of the east coast of Peninsular Malaysia. Agric. J, 1(3): 53-55.
- Ihwan, M. Z, Wahidah, W., Ambak, M. A. and Marina, H. (2016d). Report of Probopyrussp. (Isopoda: Bopyridae) infesting Macrobrachium lanchesteri from east coast of Peninsular, Malaysia. Int J contemp res rev., 7(7).
- Ihwan, M.Z., Hassan, M.D. and Ikhwanuddin, M. (2017). Epidemiological study of gregarine parasites in wild orange mud crabs, Scylla olivacea (Herbst, 1796) from Malaysian coastal waters. Postgraduate Colloquium, Series No. 9, Faculty of Veterinary Medicine, Universiti Putra Malaysia (UPM). 25-27.
- Leander, B.S., Clopton, R.E., and Keeling, P.J. (2003). Phylogeny of gregarines (Apicomplexa) as inferred from small-subunit rDNA and beta-tubulin. Int J Syst Evol Microbiol., 53: 345-354.
- Noga, E.J. (2000). Fish Disease: Diagnosis and Treatment. Department of Companion Animal and Special Species Medicine. North Carolina State University. Iowa State University Press. Raleigh, North Carolina.